Label: LUMIGAN- bimatoprost solution/ drops
- NDC Code(s): 0023-9187-01, 0023-9187-03, 0023-9187-05, 0023-9187-07
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated December 7, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
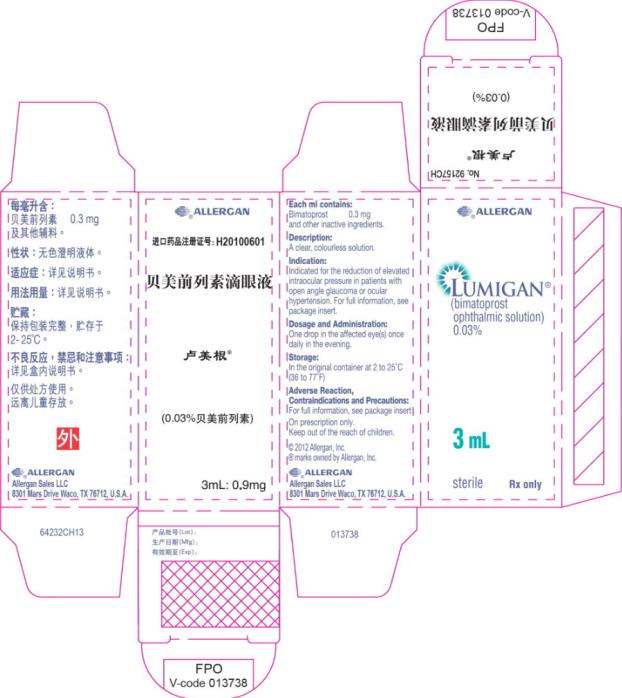
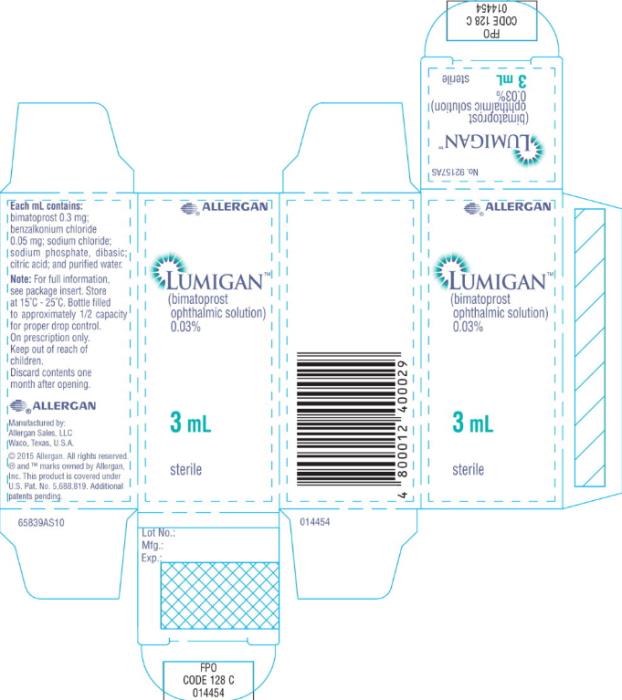
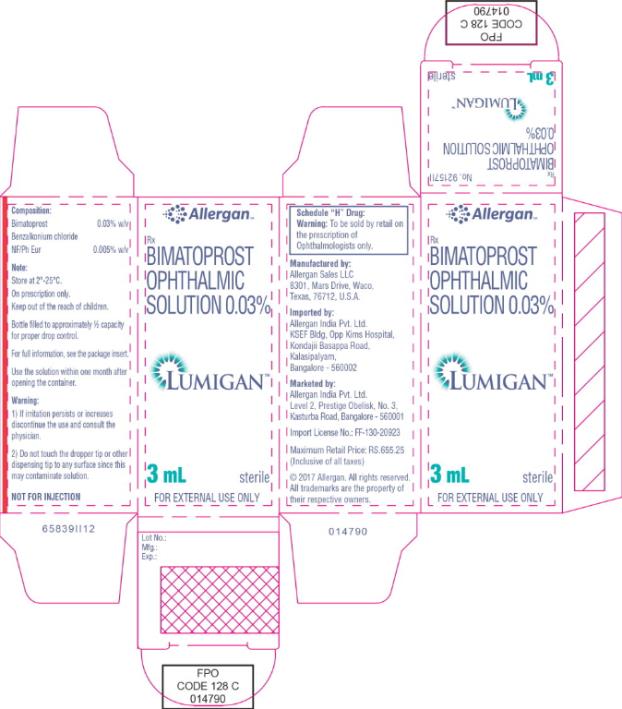
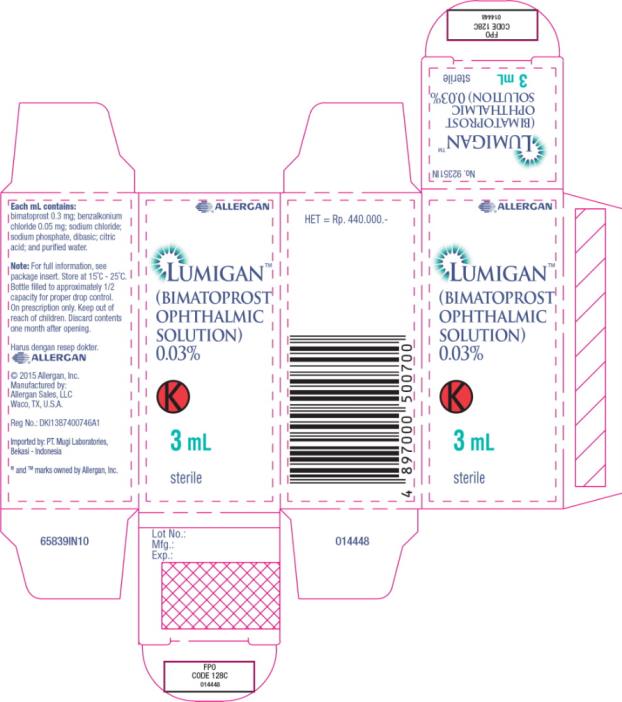
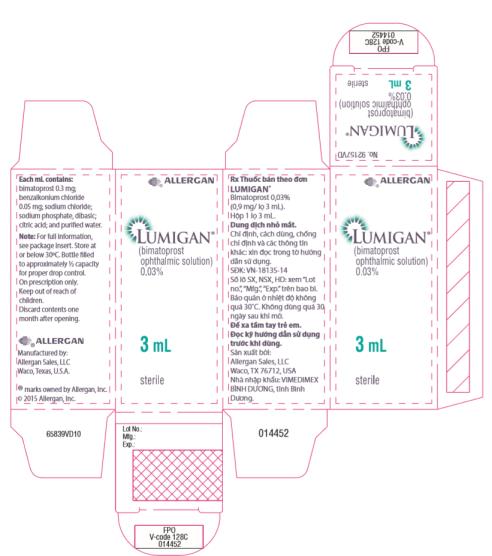
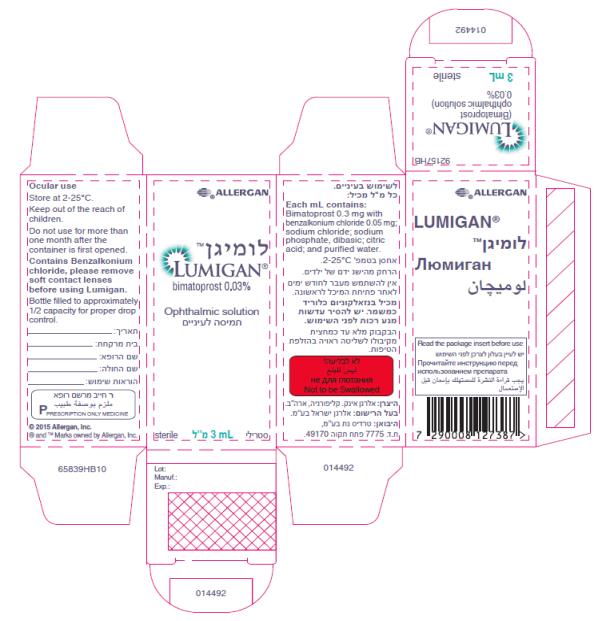
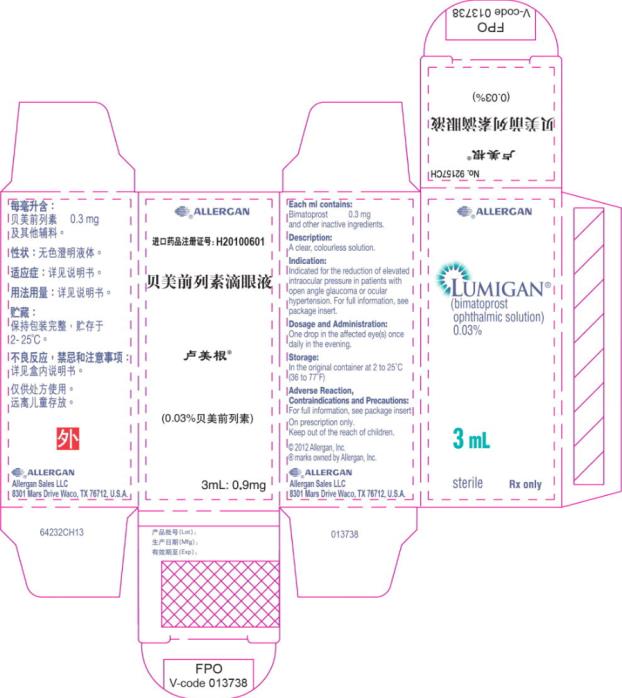
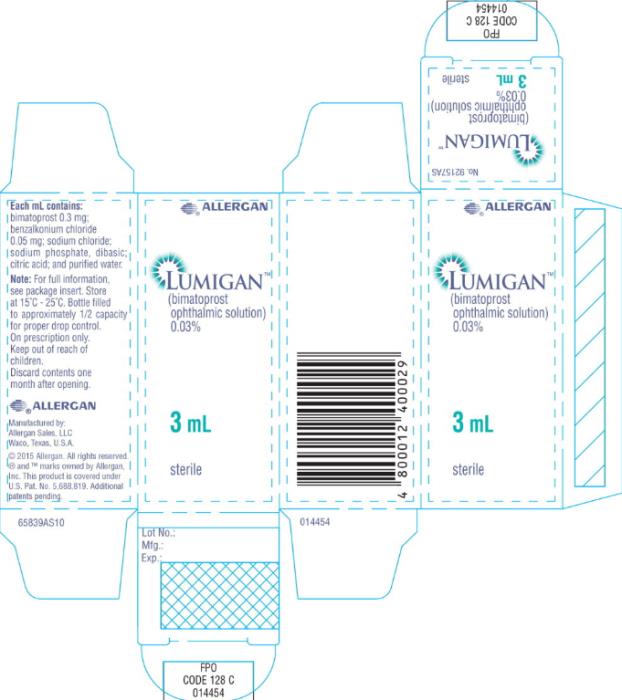
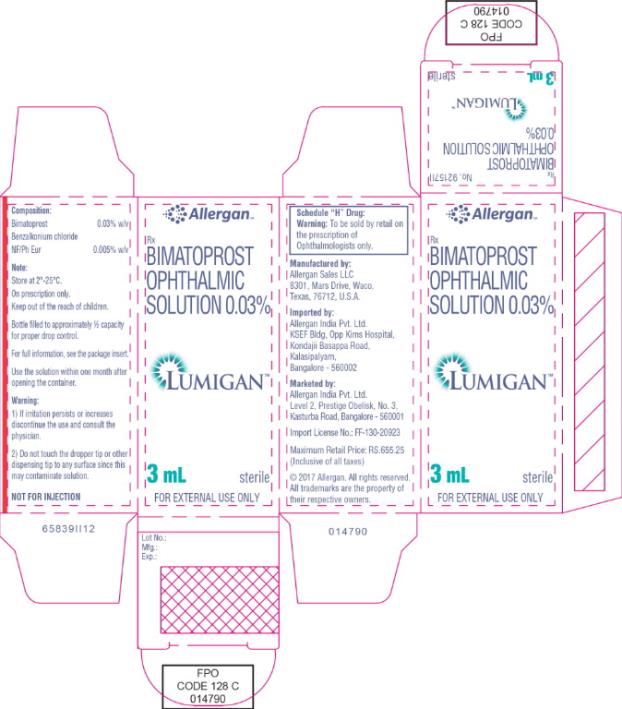
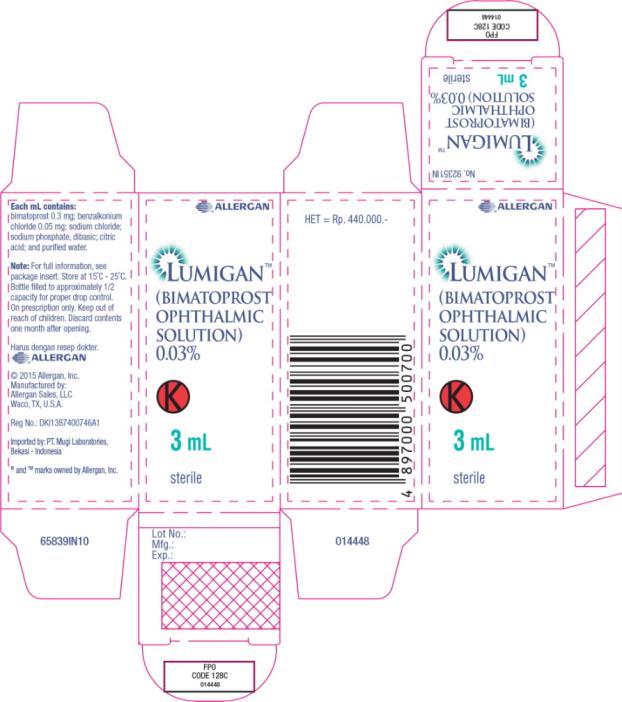
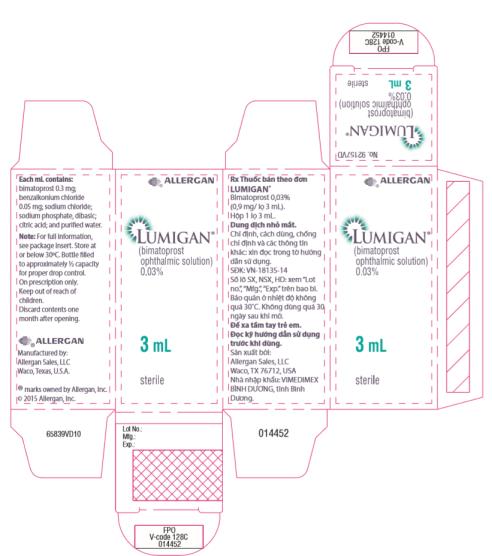
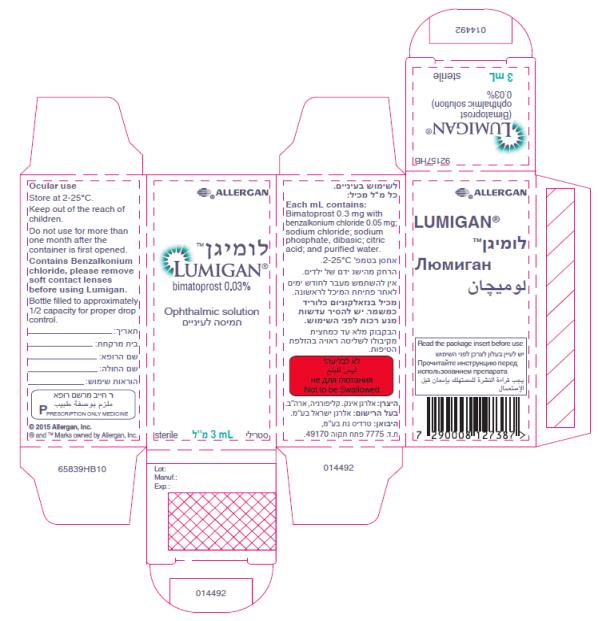
- Principal Display Panel - 0.3 mg/1 mL Carton Label
- Principal Display Panel - 0.3 mg/1 mL Carton Label
- Principal Display Panel - 0.3 mg/1 mL Carton Label
- Principal Display Panel - 0.3 mg/1 mL Carton Label
- Principal Display Panel - 0.3 mg/1 mL Carton Label
- Principal Display Panel - 0.3 mg/1 mL Carton Label
-
INGREDIENTS AND APPEARANCE
LUMIGAN
bimatoprost solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0023-9187 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BIMATOPROST (UNII: QXS94885MZ) (BIMATOPROST - UNII:QXS94885MZ) BIMATOPROST 0.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0023-9187-01 1 in 1 CARTON 03/22/2001 1 2.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:0023-9187-03 1 in 1 CARTON 03/22/2001 2 2.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 3 NDC:0023-9187-05 1 in 1 CARTON 03/22/2001 3 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 4 NDC:0023-9187-07 1 in 1 CARTON 03/22/2001 4 7.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 03/22/2001 Labeler - Allergan, Inc. (144796497)