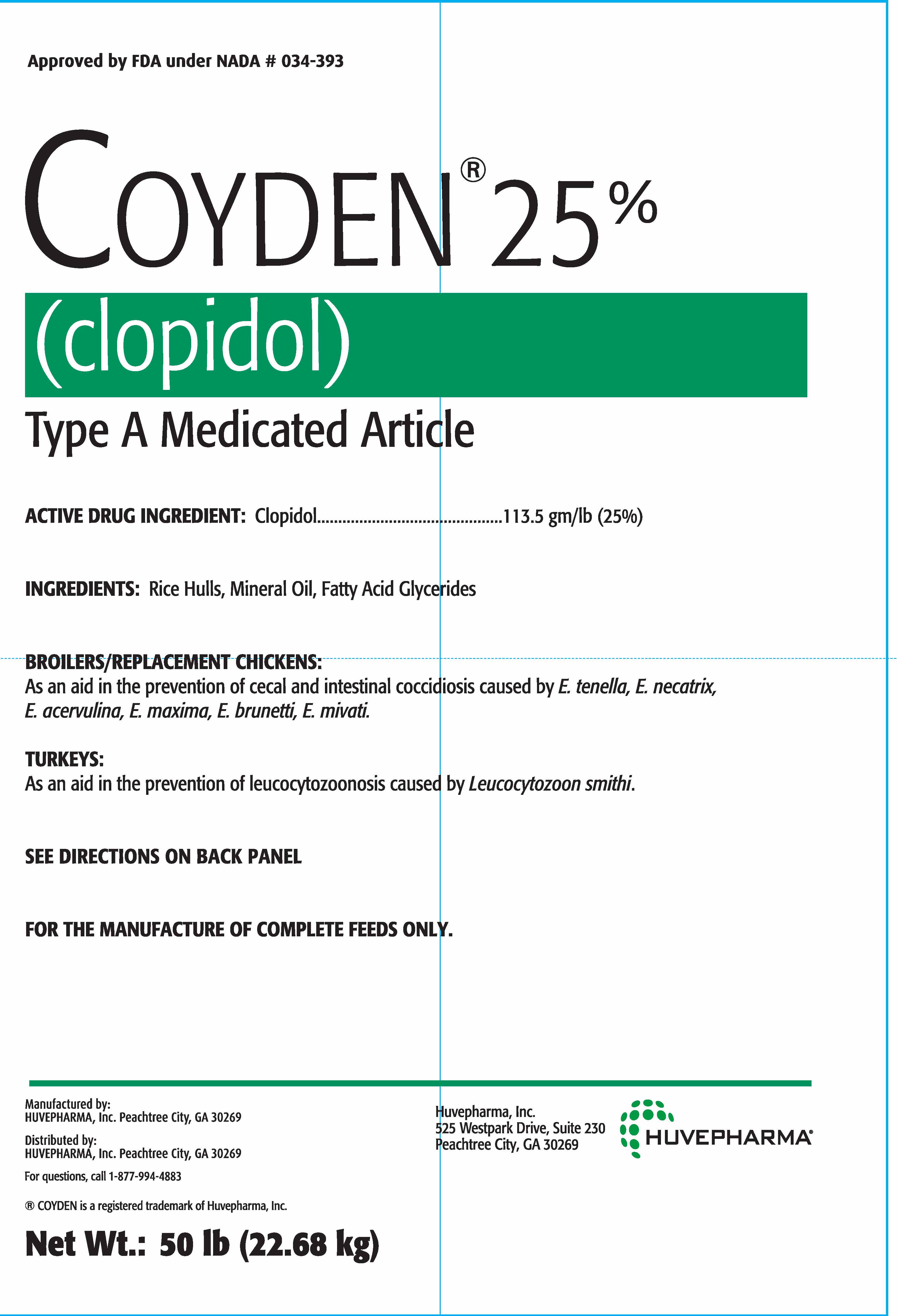
Label: COYDEN TYPE A MEDICATED ARTICLE- clopidol powder
- NDC Code(s): 23243-9568-5
- Packager: Huvepharma, Inc.
- Category: OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated February 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
-
INDICATIONS & USAGE
BROILER/REPLACEMENT CHICKENS:
As an aid in the prevention of cecal and intestinal cocciodiosis caused by E. tenella, E. necatrix,
E. acervulina, E. maxima, E. brunetti, E. mivati.TURKEYS:
As an aid in the prevention of leucocytozoonosis caused by Leucocytozoon smithi.SEE DIRECTIONS ON BACK PANEL
FOR THE MANUFACTURE OF COMPLETE FEEDS ONLY.
-
SPL UNCLASSIFIED SECTION
Manufactured by:
HUVEPHARMA, Inc. Peachtree City, GA 30269 Huvepharma, Inc.
525 Westpark Drive, Suite 230
Distributed by: Peachtree City, GA 30269
HUVEPHARMA, Inc. Peachtree City, GA 30269For questions, call 1-877-994-4883
® COYDEN is a registered trademark of Huvepharma, Inc.
Net Wt.: 50 lb (22.68 kg)
-
INDICATIONS & USAGE
INDICATIONS FOR USE:
BROILER AND REPLACEMENT CHICKENS
As an aid in the prevention of coccidiosis in broilers and replacements for caged layers caused by
E. tenella, E. necatrix, E. acervulina, E. maxima, E. brunetti, E. mivati.TURKEYS GROWN FOR MEAT PURPOSES ONLY
As an aid in the prevention of leucocytozoonosis caused by Leucocytozoon smithi. - USER SAFETY WARNINGS
- RESIDUE WARNING
- GENERAL PRECAUTIONS
-
DOSAGE & ADMINISTRATION
MIXING DIRECTIONS:
For Complete Rations - Type C Medicated Products
COYDEN® 25% (clopidol) should be thoroughly and evenly mixed in the feed in accordance with
current good manufacturing practice for medicated feed. COYDEN 25% may be used to manufacture
a Type C medicated feed in the concentration range of 0.0125% to 0.025% clopidol.To aid in even distribution of COYDEN 25% in the finished feed, prepare a mixture of COYDEN 25% in
a portion of complete feed ingredient before mixing into the finished ration. Blend the mixture with
the remainder of the finished feed and mix thoroughly.DIRECTIONS FOR USE:
Must be mixed with other ingredients before feeding.
Mixing and conveying equipment should be properly grounded.
Use 1 pound of COYDEN 25% per ton of feed to produce a feed containing 0.0125% clopidol.
Use 2 pounds of COYDEN 25% per ton of feed to produce a feed containing 0.025% clopidol.FEEDING DIRECTIONS:
Feed continuously, as the sole ration, either the 0.0125% or 0.025% Type C medicated feed
depending upon management practices, degree of exposure, and amount of feed eaten. - STORAGE AND HANDLING
- USER SAFETY WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COYDEN TYPE A MEDICATED ARTICLE
clopidol powderProduct Information Product Type OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG Item Code (Source) NDC:23243-9568 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOPIDOL (UNII: 8J763HFF5N) (CLOPIDOL - UNII:8J763HFF5N) CLOPIDOL 250.2 g in 1 kg Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) RICE BRAN (UNII: R60QEP13IC) Product Characteristics Color brown ((Light Tan to Brown)) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23243-9568-5 22.68 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA034393 05/29/2009 Labeler - Huvepharma, Inc. (619153559) Registrant - Huvepharma EOOD (552691651) Establishment Name Address ID/FEI Business Operations Huvepharma, Inc. 883128204 manufacture, analysis, pack, label, medicated animal feed manufacture Establishment Name Address ID/FEI Business Operations Jiangsu Tianhe Pharmaceutical Co., Ltd 421144690 api manufacture