Label: BEVESPI AEROSPHERE- glycopyrrolate and formoterol fumarate aerosol, metered
- NDC Code(s): 0310-4600-12, 0310-4600-28, 0310-4600-39
- Packager: AstraZeneca Pharmaceuticals LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BEVESPI AEROSPHERE safely and effectively. See full prescribing information for BEVESPI AEROSPHERE.
BEVESPI AEROSPHERE® (glycopyrrolate and formoterol fumarate) inhalation aerosol, for oral inhalation use
Initial U.S. Approval: 2016
INDICATIONS AND USAGE
BEVESPI AEROSPHERE is a combination of glycopyrrolate, an anticholinergic, and formoterol fumarate, a long-acting beta2-adrenergic agonist (LABA) indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). (1)
Limitations of Use: Not indicated for the relief of acute bronchospasm or for the treatment of asthma. (1, 5.1, 5.2)
DOSAGE AND ADMINISTRATION
- •
- For oral inhalation only.
- •
- Maintenance treatment of COPD: 2 inhalations of BEVESPI AEROSPHERE twice daily by oral inhalation. (2)
DOSAGE FORMS AND STRENGTHS
- •
- Inhalation aerosol: pressurized metered dose inhaler containing a combination of glycopyrrolate (9 mcg) and formoterol fumarate (4.8 mcg) per inhalation.
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- LABA as monotherapy (without an inhaled corticosteroid) for asthma increases the risk of serious asthma-related events. (5.1)
- •
- Do not initiate in acutely deteriorating COPD or to treat acute symptoms. (5.2)
- •
- Do not use in combination with an additional therapy containing a LABA because of risk of overdose. (5.3, 7.1)
- •
- If paradoxical bronchospasm occurs, discontinue BEVESPI AEROSPHERE and institute alternative therapy. (5.4)
- •
- Use with caution in patients with cardiovascular disorders. (5.6)
- •
- Worsening of narrow-angle glaucoma may occur. Use with caution in patients with narrow-angle glaucoma and instruct patients to contact a physician immediately if symptoms occur. (5.7)
- •
- Worsening urinary retention may occur. Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction and instruct patients to contact a physician immediately if symptoms occur. (5.8)
- •
- Use with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, and ketoacidosis. (5.9)
- •
- Be alert to hypokalemia and hyperglycemia. (5.10)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥2% and more common than with placebo) include: urinary tract infection and cough. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Other adrenergic drugs may potentiate effect: Use with caution (5.3, 7.1)
- •
- Xanthine derivatives, steroids, diuretics or non-potassium sparing diuretics may potentiate hypokalemia or ECG changes. Use with caution. (7.2, 7.3)
- •
- Diuretics: Use with caution. Electrocardiographic changes and/or hypokalemia associated with non-potassium sparing diuretics may worsen with concomitant beta2-agonists. (7.3)
- •
- Monoamine oxidase inhibitors and tricyclic antidepressants: Use with extreme caution. May potentiate effect of formoterol fumarate on cardiovascular system. (7.4)
- •
- Beta-blockers: Use with caution and only when medically necessary. (7.5)
- •
- Anticholinergics: May interact additively with concomitantly used anticholinergic medications. Avoid administrations of BEVESPI AEROSPHERE with other anticholinergic-containing drugs. (7.6)
USE IN SPECIFIC POPULATIONS
- •
- In patients with severe renal impairment use should be considered only if the potential benefit of the treatment outweighs the risk. (8.7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Administration
2.2 Preparation
2.3 Dose Counter
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
5.2 Deterioration of Disease and Acute Episodes
5.3 Avoid Excessive Use of BEVESPI and Avoid Use with Other Long-Acting Beta2-Agonists
5.4 Paradoxical Bronchospasm
5.5 Hypersensitivity Reactions including Anaphylaxis
5.6 Cardiovascular Effects
5.7 Worsening of Narrow-Angle Glaucoma
5.8 Worsening of Urinary Retention
5.9 Coexisting Conditions
5.10 Hypokalemia and Hyperglycemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Adrenergic Drugs
7.2 Xanthine Derivatives, Steroids, or Diuretics
7.3 Non-Potassium Sparing Diuretics
7.4 Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, QTc Prolonging Drugs
7.5 Beta-Blockers
7.6 Anticholinergics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Dose-Ranging Trials
14.2 Confirmatory Trials
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Administration
The recommended dosage of BEVESPI AEROSPHERE is glycopyrrolate 18 mcg and formoterol fumarate 9.6 mcg (administered as two inhalations of BEVESPI AEROSPHERE [glycopyrrolate/formoterol fumarate 9 mcg/4.8 mcg]) twice daily in the morning and in the evening by oral inhalation. Do not take more than two inhalations twice daily.
2.2 Preparation
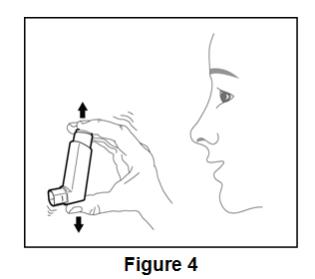
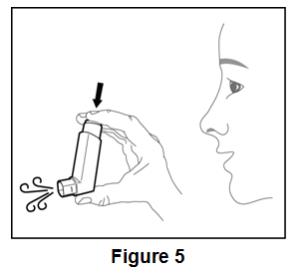
Prime BEVESPI AEROSPHERE before using for the first time. Priming BEVESPI AEROSPHERE is essential to ensure appropriate drug content in each actuation. To prime BEVESPI AEROSPHERE, release 4 sprays into the air away from the face, shaking well before each spray. BEVESPI AEROSPHERE must be re-primed when the inhaler has not been used for more than 7 days. To re-prime BEVESPI AEROSPHERE, release 2 sprays into the air away from the face, shaking well before each spray.
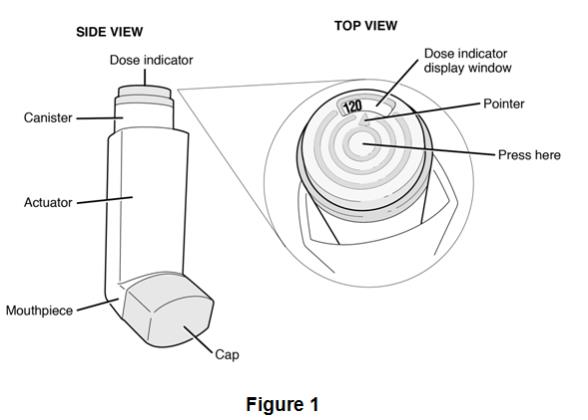
2.3 Dose Counter
The canister has an attached dose indicator, which indicates how many inhalations remain. The dose indicator display will move after every tenth actuation. When nearing the end of the usable inhalations, the color behind the number in the dose indicator display window changes to red. BEVESPI AEROSPHERE should be discarded when the dose indicator display window shows zero.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
BEVESPI AEROSPHERE is contraindicated in:
- •
- use of a long-acting beta2-adrenergic agonist (LABA), including formoterol fumarate, one of the active ingredients in BEVESPI AEROSPHERE, without an inhaled corticosteroid, in patients with asthma [see Warnings and Precautions (5.1)]. BEVESPI AEROSPHERE is not indicated for the treatment of asthma.
- •
- patients with hypersensitivity to glycopyrrolate, formoterol fumarate, or to any component of the product [see Warnings and Precautions (5.5)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
- •
- The safety and efficacy of BEVESPI AEROSPHERE in patients with asthma have not been established. BEVESPI AEROSPHERE is not indicated for the treatment of asthma [see Contraindications (4)].
- •
- Use of LABA as monotherapy [without inhaled corticosteroids (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone.
- •
- A 28-week, placebo-controlled US trial comparing the safety of another LABA (salmeterol) with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in subjects receiving salmeterol (13/13,176 in subjects treated with salmeterol vs. 3/13,179 in subjects treated with placebo; RR 4.37, 95% CI: 1.25, 15.34). The increased risk of asthma-related death is considered a class effect of LABAs, including formoterol fumarate, one of the active ingredients in BEVESPI AEROSPHERE.
- •
- No trial adequate to determine whether the rate of asthma-related deaths is increased in patients treated with BEVESPI AEROSPHERE has been conducted.
- •
- Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
5.2 Deterioration of Disease and Acute Episodes
BEVESPI AEROSPHERE should not be initiated in patients with acutely deteriorating COPD, which may be a life-threatening condition. BEVESPI AEROSPHERE has not been studied in patients with acutely deteriorating COPD. The use of BEVESPI AEROSPHERE in this setting is inappropriate.
BEVESPI AEROSPHERE should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. BEVESPI AEROSPHERE has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled short-acting beta2-agonist.
When beginning BEVESPI AEROSPHERE, patients who have been taking inhaled, short-acting beta2-agonists on a regular basis (e.g., four times a day) should be instructed to discontinue the regular use of these medicines and use them only for symptomatic relief of acute respiratory symptoms. When prescribing BEVESPI AEROSPHERE, the healthcare provider should also prescribe an inhaled, short acting beta2-agonist and instruct the patient on how it should be used. Increasing inhaled beta2-agonist use is a signal of deteriorating disease for which prompt medical attention is indicated.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If BEVESPI AEROSPHERE no longer controls the symptoms of bronchoconstriction, or the patient’s inhaled, short-acting beta2-agonist becomes less effective, or the patient needs more inhalations of short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dosage of BEVESPI AEROSPHERE beyond the recommended dose is not appropriate in this situation.
5.3 Avoid Excessive Use of BEVESPI and Avoid Use with Other Long-Acting Beta2-Agonists
As with other inhaled medicines containing beta2-agonists, BEVESPI AEROSPHERE should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing LABAs, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic medicines. Patients using BEVESPI AEROSPHERE should not use another medicine containing a LABA for any reason [see Drug Interactions (7.1)].
5.4 Paradoxical Bronchospasm
As with other inhaled medicines, BEVESPI AEROSPHERE can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with BEVESPI AEROSPHERE, it should be treated immediately with an inhaled, short-acting bronchodilator, BEVESPI AEROSPHERE should be discontinued immediately, and alternative therapy should be instituted.
5.5 Hypersensitivity Reactions including Anaphylaxis
Immediate hypersensitivity reactions have been reported after administration of glycopyrrolate or formoterol fumarate, the components of BEVESPI AEROSPHERE. If signs suggesting allergic reactions occur, in particular, angioedema (including difficulties in breathing or swallowing, swelling of tongue, lips, and face), urticaria, or skin rash, BEVESPI AEROSPHERE should be stopped at once and alternative treatment should be considered.
5.6 Cardiovascular Effects
Formoterol fumarate, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, or symptoms [see Clinical Pharmacology (12.2)]. If such effects occur, BEVESPI AEROSPHERE may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiographic changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression, although the clinical significance of these findings is unknown.
Therefore, BEVESPI AEROSPHERE should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
5.7 Worsening of Narrow-Angle Glaucoma
BEVESPI AEROSPHERE should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos, or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
5.8 Worsening of Urinary Retention
BEVESPI AEROSPHERE should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
5.9 Coexisting Conditions
BEVESPI AEROSPHERE, like all medications containing sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis and in those who are unusually responsive to sympathomimetic amines. Doses of the related beta2-agonist albuterol, when administered intravenously, have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis.
5.10 Hypokalemia and Hyperglycemia
Beta2-agonist medications may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects [see Clinical Pharmacology (12.2)]. The decrease in serum potassium is usually transient, not requiring supplementation. Beta2-agonist medicines may produce transient hyperglycemia in some patients. In two clinical trials of 24-weeks and a 28-week safety extension study evaluating BEVESPI AEROSPHERE in subjects with COPD, there was no evidence of a treatment effect on serum glucose or potassium.
-
6 ADVERSE REACTIONS
The following adverse reactions are described in greater detail elsewhere in the labeling:
- •
- Paradoxical bronchospasm [see Warnings and Precautions (5.4)]
- •
- Hypersensitivity reactions including Anaphylaxis [see Contraindications (4), Warnings and Precautions (5.5)]
- •
- Cardiovascular effects [see Warnings and Precautions (5.6)]
- •
- Worsening of narrow-angle glaucoma [see Warnings and Precautions (5.7)]
- •
- Worsening of urinary retention [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The clinical program for BEVESPI AEROSPHERE included 4,911 subjects with COPD in two 24-week lung function trials, one long-term safety extension study of 28 weeks, and 10 other trials of shorter duration. A total of 1,302 subjects have received at least 1 dose of BEVESPI AEROSPHERE. The safety data described below are based on the two 24-week trials and the one 28-week long-term safety extension trial. Adverse reactions observed in the other trials were similar to those observed in these confirmatory trials.
24-Week Trials
The incidence of adverse reactions with BEVESPI AEROSPHERE in Table 1 is based on reports in two 24-week, placebo-controlled trials (Trials 1 and 2; n=2,100 and n=1,610, respectively). Of the 3,710 subjects, 56% were male and 91% were Caucasian. They had a mean age of 63 years and an average smoking history of 51 pack-years, with 54% identified as current smokers. At screening, the mean post-bronchodilator percent predicted forced expiratory volume in 1 second (FEV1) was 51% (range: 19% to 82%) and the mean percent reversibility was 20% (range: -32% to 135%).
Subjects received one of the following treatments: BEVESPI AEROSPHERE, glycopyrrolate 18 mcg, formoterol fumarate 9.6 mcg, or placebo twice daily or active control.
Table 1 - Adverse Reactions with BEVESPI AEROSPHERE ≥2% Incidence and More
Common than with Placebo in Subjects with Chronic Obstructive Pulmonary DiseaseAdverse Reaction
BEVESPI AEROSPHERE
(n=1036)
%Glycopyrrolate
18 mcg BID
(n=890)
%Formoterol Fumarate
9.6 mcg BID
(n=890)
%
Placebo
(n=443)
%Respiratory, thoracic, and mediastinal disorders
Cough
4.0
3.0
2.7
2.7
Infections and infestation
Urinary tract infection
2.6
1.8
1.5
2.3
Other adverse reactions defined as events with an incidence of >1% but less than 2% with BEVESPI AEROSPHERE but more common than with placebo included the following: arthralgia, chest pain, tooth abscess, muscle spasms, headache, oropharyngeal pain, vomiting, pain in extremity, dizziness, anxiety, dry mouth, fall, influenza, fatigue, acute sinusitis, and contusion.
Long-Term Safety Extension Trial
In a 28-week long-term safety extension trial, 893 subjects who successfully completed Trial 1 or Trial 2 were treated for up to an additional 28 weeks for a total treatment period of up to 52 weeks with BEVESPI AEROSPHERE, glycopyrrolate 18 mcg, formoterol fumarate 9.6 mcg administered twice daily or active control. Because the subjects continued from Trial 1 or Trial 2 into the safety extension trial, the demographic and baseline characteristics of the long-term safety extension trial were similar to those of the placebo-controlled efficacy trials described above. The adverse reactions reported in the long-term safety trial were consistent with those observed in the 24-week placebo-controlled trials.
Additional Adverse Reactions: Other adverse reactions that have been associated with the component formoterol fumarate include: hypersensitivity reactions, hyperglycemia, sleep disturbance, agitation, restlessness, tremor, nausea, tachycardia, palpitations, cardiac arrhythmias (atrial fibrillation, supraventricular tachycardia, and extrasystoles).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of BEVESPI AEROSPHERE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In postmarketing experience with BEVESPI AEROSPHERE, hypersensitivity and urinary retention have been reported.
-
7 DRUG INTERACTIONS
No formal drug interaction studies have been performed with BEVESPI AEROSPHERE.
7.1 Adrenergic Drugs
If additional adrenergic drugs are to be administered by any route, they should be used with caution because the sympathetic effects of formoterol, a component of BEVESPI AEROSPHERE, may be potentiated [see Warnings and Precautions (5.3)].
7.2 Xanthine Derivatives, Steroids, or Diuretics
Concomitant treatment with xanthine derivatives, steroids, or diuretics may potentiate any hypokalemic effect of beta2 adrenergic agonists such as formoterol, a component of BEVESPI AEROSPHERE.
7.3 Non-Potassium Sparing Diuretics
The ECG changes and/or hypokalemia that may result from the administration of non-potassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta2-agonists, especially when the recommended dose of the beta2-agonist is exceeded. Approximately 17% of subjects were taking non-potassium sparing diuretics during the two 24-week placebo-controlled trials in subjects with COPD. The incidence of adverse events in subjects taking non-potassium-sparing diuretics was similar between BEVESPI AEROSPHERE and placebo treatment groups. In addition, there was no evidence of a treatment effect on serum potassium with BEVESPI AEROSPHERE compared to placebo in subjects taking non-potassium sparing diuretics during the two 24-week trials. However, caution is advised in the coadministration of BEVESPI AEROSPHERE with non-potassium-sparing diuretics.
7.4 Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, QTc Prolonging Drugs
BEVESPI AEROSPHERE, as with other beta2-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants or other drugs known to prolong the QTc interval because the action of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval may be associated with an increased risk of ventricular arrhythmias.
7.5 Beta-Blockers
Beta-adrenergic receptor antagonists (beta-blockers) and BEVESPI AEROSPHERE may interfere with the effect of each other when administered concurrently. Beta-blockers not only block the therapeutic effects of beta2-agonists, but may produce severe bronchospasm in COPD patients. Therefore, patients with COPD should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-blockers in patients with COPD. In this setting, cardioselective beta-blockers could be considered, although they should be administered with caution.
7.6 Anticholinergics
There is a potential for an additive interaction with concomitantly used anticholinergic medications. Therefore, avoid coadministration of BEVESPI AEROSPHERE with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects [see Warnings and Precautions (5.7, 5.8) and Adverse Reactions (6)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled trials of BEVESPI AEROSPHERE or its individual components, glycopyrrolate and formoterol fumarate, in pregnant women to inform a drug-associated risk.
In animal reproduction studies, glycopyrrolate alone, administered by the subcutaneous route in rats and rabbits, did not cause structural abnormalities or affect fetal survival at exposures approximately 2700 and 5400 times from the maximum recommended human daily inhalation dose (MRHDID), respectively. Glycopyrrolate had no effects on the physical, functional, and behavioral development of rat pups with exposures up to 2700 times the MRHDID.
Formoterol fumarate alone, administered by the oral route in rats and rabbits, caused structural abnormalities at 1500 and 61,000 times the MRHDID, respectively. Formoterol fumarate was also embryocidal, increased pup loss at birth and during lactation, and decreased pup weight in rats at 110 times the MRHDID. These adverse effects generally occurred at large multiples of the MRHDID when formoterol fumarate was administered by the oral route to achieve high systemic exposures. No structural abnormalities, embryocidal, or developmental effects were seen in rats that received inhalation doses up to 350 times the MRHDID.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Labor or Delivery: There are no well-controlled human trials that have investigated the effects of BEVESPI AEROSPHERE on preterm labor or labor at term. Because beta2-agonists may potentially interfere with uterine contractility, BEVESPI AEROSPHERE should be used during labor only if the potential benefit justifies the potential risk.
Data
Animal Data
Glycopyrrolate
In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 6 to 17, glycopyrrolate produced no structural abnormalities or effects on fetal survival; however, slight reductions of fetal body weight in the presence of maternal toxicity at the highest tested dose that was 2700 times the MRHDID (on a mcg/m2 basis at a maternal subcutaneous dose of 10,000 mcg/kg/day). Fetal body weights were unaffected with doses up to 270 times the MRHDID (on a mcg/m2 basis with maternal subcutaneous doses up to 1000 mcg/kg/day). Maternal toxicity was observed with doses 270 times the MRHDID and higher (on a mcg/m2 basis with maternal subcutaneous doses of 1000 mcg/kg/day and higher).
In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 6 to 18, glycopyrrolate produced no structural abnormalities or effects on fetal survival; however, slight reductions of fetal body weight in the presence of maternal toxicity at the highest tested dose that was 5400 times the MRHDID (on a mcg/m2 basis at a maternal subcutaneous dose of 10,000 mcg/kg/day). Fetal body weights were unaffected with doses up to 540 times the MRHDID (on a mcg/m2 basis with maternal subcutaneous doses up to 1000 mcg/kg/day). Maternal toxicity was observed with doses 540 times the MRHDID and higher (on a mcg/m2 basis with maternal subcutaneous doses of 1000 mcg/kg/day and higher).
In a pre- and post-natal development study, pregnant female rats received glycopyrrolate at doses of 100, 1000, and 10,000 mcg/kg/day from gestation day 6 through the lactation period. Pup body weight gain was slightly reduced from birth through the lactation period at a dose 2700 times the MRHDID (on a mcg/m2 basis with a maternal subcutaneous dose of 10,000 mcg/kg/day); however, pup body weight gain was unaffected after weaning There were no treatment-related effects on the physical, functional, and behavioral development of pups with doses up to 2700 times the MRHDID (on a mcg/m2 basis with maternal subcutaneous doses up to 10,000 mcg/kg/day). Maternal toxicity was observed from gestation days 6 to 18 with doses 270 times the MRHDID and higher (on a mcg/m2 basis with maternal subcutaneous doses of 1000 mcg/kg/day and higher).
Formoterol Fumarate
In a fertility and reproduction study, male rats were orally dosed for at least 9 weeks and females for 2 weeks prior to pairing and throughout the mating period. Females were either dosed up to gestation day 19 or up until weaning of their offspring. Males were dosed up to 25 weeks. Umbilical hernia was observed in rat fetuses at oral doses 1500 times the MRHDID (on a mcg/m2 basis at maternal oral doses of 3000 mcg/kg/day and higher). Brachygnathia was observed in rat fetuses at a dose 8000 times the MRHDID (on a mcg/m2 basis at a maternal oral dose of 15,000 mcg/kg/day). Pregnancy was prolonged at a dose 8000 times the MRHDID (on a mcg/m2 basis at a maternal oral dose of 15,000 mcg/kg/day). Fetal and pup deaths occurred at doses approximately 1500 times the MRHDID and higher (on a mcg/m2 basis at oral doses of 3000 mcg/kg/day and higher) during gestation.
In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis, no structural abnormalities, embryocidal effects, or developmental effects were seen at doses up to 350 times the MRHDID (on a mcg/m2 basis with maternal inhalation doses up to 690 mcg/kg/day).
In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 6 to 18, subcapsular cysts on the liver were observed in the fetuses at a dose 61,000 times the MRHDID (on a mcg/m2 basis with a maternal oral dose of 60,000 mcg/kg/day). No teratogenic effects were observed at doses up to 3500 times the MRHDID (on a mcg/m2 basis at maternal oral doses up to 3500 mcg/kg/day).
In a pre- and post-natal development study, pregnant female rats received formoterol at oral doses of 0, 210, 840, and 3400 mcg/kg/day from gestation day 6 (completion of implantation) through the lactation period. Pup survival was decreased from birth to postpartum day 26 at doses 110 times the MRHDID and higher (on a mcg/m2 basis at maternal oral doses of 210 mcg/kg/day and higher), although there was no evidence of a dose-response relationship. There were no treatment-related effects on the physical, functional, and behavioral development of rat pups.
8.2 Lactation
Risk Summary
There are no available data on the effects of BEVESPI AEROSPHERE, glycopyrrolate, or formoterol fumarate on the breastfed child or on milk production. There are no available data on the presence of glycopyrrolate or formoterol fumarate in human milk. Formoterol fumarate and glycopyrrolate have been detected in the plasma of undosed rat pups suckling from exposed dams [see Data]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for BEVESPI AEROSPHERE and any potential adverse effects on the breast-fed child from BEVESPI AEROSPHERE or from the underlying maternal condition.
Animal Data
In the reproductive/developmental toxicity study in rats, plasma levels of glycopyrrolate were measured in pups on post-natal day 4. The maximum concentration in the pups was 6% of the maternal dose of 10 mg/kg/day (pup plasma concentration of 96 ng/mL at 1 hour after dosing corresponded with 1610 ng/mL in the dam at 0.5 hours after dosing).
In the fertility and reproduction study in rats, plasma levels of formoterol were measured in pups on post-natal day 15 [see Use in Specific Populations (8.1)]. It was estimated that the maximum plasma concentration that the pups received from the maternal animal, at the highest dose of 15 mg/kg, after nursing was 4.4% (0.24 nmol/L for a litter vs. 5.5 nmol/L for the mother).
8.4 Pediatric Use
BEVESPI AEROSPHERE is not indicated for use in children. The safety and effectiveness of BEVESPI AEROSPHERE in the pediatric population have not been established.
8.5 Geriatric Use
Based on available data, no adjustment of the dosage of BEVESPI AEROSPHERE in geriatric patients is necessary, but greater sensitivity in some older individuals cannot be ruled out.
The confirmatory trials of BEVESPI AEROSPHERE for COPD included 1,680 subjects aged 65 and older and, of those, 290 subjects were aged 75 and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
8.6 Hepatic Impairment
Formal pharmacokinetic studies using BEVESPI AEROSPHERE have not been conducted in patients with hepatic impairment. However, since formoterol fumarate is predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of formoterol fumarate in plasma. Therefore, patients with hepatic disease should be closely monitored.
8.7 Renal Impairment
Formal pharmacokinetic studies using BEVESPI AEROSPHERE have not been conducted in patients with renal impairment. In patients with severe renal impairment (creatinine clearance of ≤30 mL/min/1.73 m2) or end-stage renal disease requiring dialysis, use of BEVESPI AEROSPHERE should be considered only if the expected benefit outweighs the potential risk [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
No cases of overdose have been reported with BEVESPI AEROSPHERE. BEVESPI AEROSPHERE contains both glycopyrrolate and formoterol fumarate; therefore, the risks associated with overdosage for the individual components described below apply to BEVESPI AEROSPHERE. Treatment of overdosage consists of discontinuation of BEVESPI AEROSPHERE together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. Cardiac monitoring is recommended in case of overdosage.
Glycopyrrolate
High doses of glycopyrrolate, a component of BEVESPI AEROSPHERE, may lead to anticholinergic signs and symptoms such as nausea, vomiting, dizziness, lightheadedness, blurred vision, increased intraocular pressure (causing pain, vision disturbances or reddening of the eye), obstipation or difficulties in voiding.
Formoterol Fumarate
An overdose of formoterol fumarate would likely lead to an exaggeration of effects that are typical for beta2-agonists: seizures, angina, hypertension, hypotension, tachycardia, atrial and ventricular tachyarrhythmias, nervousness, headache, tremor, palpitations, muscle cramps, nausea, dizziness, sleep disturbances, metabolic acidosis, hyperglycemia, hypokalemia. As with all sympathomimetic medications, cardiac arrest and even death may be associated with overdosage of formoterol fumarate.
-
11 DESCRIPTION
BEVESPI AEROSPHERE (glycopyrrolate and formoterol fumarate) Inhalation Aerosol is a pressurized metered-dose inhaler that contains a combination of micronized glycopyrrolate, an anticholinergic, and micronized formoterol fumarate, a long-acting beta2-adrenergic agonist, for oral inhalation.
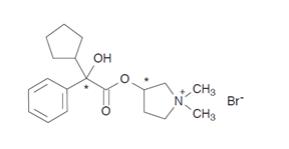
Glycopyrrolate is a quaternary ammonium salt with the following chemical name: (RS)-[3-(SR)-Hydroxy-1,1-dimethylpyrrolidinium bromide] α-cyclopentylmandelate. Glycopyrrolate is a powder that is freely soluble in water. The molecular formula is C19H28BrNO3, and the molecular weight is 398.33 g/mol. The structural formula is as follows:
Glycopyrrolate contains two chiral centers (denoted by * in structure above) and is a racemate of a 1:1 mixture of the R,S and S,R diastereomers. The active moiety, glycopyrronium, is the positively charged ion of glycopyrrolate.
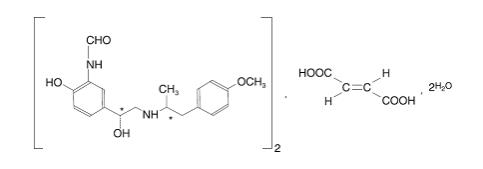
Formoterol fumarate has the chemical name N-[2-Hydroxy-5-[(1RS)-1-hydroxy-2-[[(1RS)-2-(4-methoxyphenyl)-1- methylethyl]-amino] ethyl]phenyl] formamide, €-2-butenedioate dihydrate. Formoterol fumarate is a powder that is slightly soluble in water. The molecular formula is (C19H24N2O4)2.C4H4O4.2H2O and the molecular weight is 840.91 g/mol. The structural formula is as follows:
Formoterol fumarate contains two chiral centers (denoted by * in structure above), and consists of a single enantiomeric pair (a racemate of R,R and S,S).
BEVESPI AEROSPHERE is formulated as a hydrofluoroalkane (HFA 134a) propelled pressurized metered dose inhaler containing 120 inhalations. The canister has an attached dose indicator and is supplied with a white plastic actuator body and mouthpiece with an orange dust cap.
After priming each actuation of the inhaler meters 10.4 mcg of glycopyrrolate (equivalent to 8.3 mcg of glycopyrronium) and 5.5 mcg of formoterol fumarate from the valve which delivers 9 mcg of glycopyrrolate (equivalent to 7.2 mcg of glycopyrronium) and 4.8 mcg of formoterol fumarate from the actuator. The actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between actuation of the device and inspiration through the delivery system. BEVESPI AEROSPHERE also contains porous particles that form a cosuspension with the drug crystals. The porous particles are comprised of the phospholipid, 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC), and calcium chloride. Porous particles and HFA 134a are excipients in the formulation.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
BEVESPI AEROSPHERE
BEVESPI AEROSPHERE contains both glycopyrrolate and formoterol fumarate. The mechanism of action described below for the individual components applies to BEVESPI AEROSPHERE. These drugs represent two different classes of medications (an anticholinergic, and a long-acting selective beta2-adrenoceptor agonist) that have different effects on clinical physiology and inflammatory indices of COPD.
Glycopyrrolate
Glycopyrrolate is a long-acting antimuscarinic agent which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of the M3 receptor at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methylcholine and acetylcholine-induced bronchoconstrictive effects was dose-dependent and lasted more than 12 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of glycopyrrolate is predominantly a site-specific effect.
Formoterol Fumarate
Formoterol fumarate is a long-acting selective beta2-adrenergic agonist (beta2-agonist) with a rapid onset of action. Inhaled formoterol fumarate acts locally in the lung as a bronchodilator. In vitro studies have shown that formoterol has more than 200-fold greater agonist activity at beta2-receptors than at beta1-receptors. The in vitro binding selectivity to beta2- over beta1-adrenoceptors is higher for formoterol than for albuterol (5 times), whereas salmeterol has a higher (3 times) beta2-selectivity ratio than formoterol.
Although beta2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-receptors are the predominant receptors in the heart, there are also beta2-receptors in the human heart comprising 10% to 50% of the total beta-adrenergic receptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta2-agonists may have cardiac effects.
The pharmacologic effects of beta2-adrenoceptor agonist drugs, including formoterol fumarate, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3', 5'-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The potential for QTc interval prolongation was assessed in a double-blind, single-dose, placebo- and positive-controlled crossover trial in 69 healthy subjects. The largest mean (90% upper confidence bound) differences from placebo in baseline-corrected QTcI for 2 inhalations of BEVESPI AEROSPHERE and glycopyrrolate/formoterol fumarate 72/19.2 mcg, were 3.1 (4.7) ms and 7.6 (9.2) ms, respectively, and excluded the clinically relevant threshold of 10 ms.
A dose-dependent increase in heart rate was also observed. The largest mean (90% upper confidence bound) differences from placebo in baseline-corrected heart rate were 3.3 (4.9) beats/min and 7.6 (9.5) beats/min seen within 10 minutes of dosing with 2 inhalations of BEVESPI AEROSPHERE and glycopyrrolate/formoterol fumarate 72/19.2 mcg, respectively.
Chronic Obstructive Pulmonary Disease
The effect of glycopyrrolate/formoterol fumarate on cardiac rhythm in subjects with COPD was assessed using 24-hour Holter monitoring in 2-week and 24-week trials. All treatments were administered as two inhalations twice daily. In the 2-week trial, the Holter monitoring population included 58 subjects on glycopyrrolate/formoterol fumarate 18/4.8 mcg, 58 subjects on glycopyrrolate 18 mcg, and 60 subjects on formoterol fumarate 4.8 mcg. In the 24-week trial, the Holter monitoring population included 171 subjects on BEVESPI AEROSPHERE, 160 subjects on glycopyrrolate 9 mcg, 174 subjects on formoterol fumarate 4.8 mcg, and 80 subjects on placebo. No clinically meaningful effects on cardiac rhythm were observed.
12.3 Pharmacokinetics
Linear pharmacokinetics were observed for glycopyrrolate (dose range: 18 to 144 mcg) and formoterol fumarate (dose range: 2.4 to 19.2 mcg) after oral inhalation.
Absorption
Glycopyrrolate: Following inhaled administration of BEVESPI AEROSPHERE in subjects with COPD, Cmax occurred at 5 minutes. Steady state is expected to be achieved within 2-3 days of repeated dosing of BEVESPI AEROSPHERE and the extent of exposure is approximately 2.3 times higher than after the first dose.
Formoterol Fumarate: Following inhaled administration of BEVESPI AEROSPHERE in subjects with COPD, Cmax occurred within 20 to 60 minutes. Steady state is expected to be achieved within 2-3 days of repeated dosing with BEVESPI AEROSPHERE and the extent of exposure is approximately 1.5 times higher than after the first dose.
Distribution
Glycopyrrolate: Population pharmacokinetic analysis showed that estimated Vc/F (volume of the central compartment), and V2/F (volume of the peripheral compartment) are 951 L, and 2019 L, respectively.
Formoterol Fumarate: Population pharmacokinetic analysis showed that estimated Vc/F (volume of the central compartment), and V2/F (volume of the peripheral compartment) are 948 L, and 434 L, respectively. Over the concentration range of 10-500 nmol/L, plasma protein binding of formoterol ranged from 46% to 58%.
Elimination
Glycopyrrolate: After IV administration of a 0.2 mg dose of radiolabeled glycopyrrolate, 85% of the dose recovered was recovered in urine 48 hours post dose and some of radioactivity was also recovered in bile. The terminal elimination half-life derived via population pharmacokinetics analysis was 11.8 hours.
Formoterol Fumarate: The excretion of formoterol was studied in four healthy subjects following simultaneous administration of radiolabeled formoterol via the oral and IV routes. In that study, 62% of the radiolabeled formoterol was excreted in the urine while 24% was eliminated in the feces. The terminal elimination half-life derived via population pharmacokinetics analysis was 11.8 hours.
Metabolism
Glycopyrrolate: Based on literature, and an in-vitro human hepatocyte study, metabolism plays a minor role in the overall elimination of glycopyrronium. CYP2D6 was found to be the predominant enzyme involved in the metabolism of glycopyrronium. In-vitro studies indicate the glycopyrrolate does not inhibit any subtype of cytochrome P450 and that there is no induction of CYP1A2, 2B6, or 3A4 at therapeutically relevant concentrations.
Formoterol Fumarate: The primary metabolism of formoterol is by direct glucuronidation and by O-demethylation followed by conjugation to inactive metabolites. Secondary metabolic pathways include deformylation and sulfate conjugation. CYP2D6 and CYP2C have been identified as being primarily responsible for O-demethylation.
Specific Populations
Population pharmacokinetic analysis showed no evidence of a clinically significant effect of age, sex, race/ethnicity, or body weight on the pharmacokinetics of glycopyrrolate and formoterol.
Patients with Hepatic Impairment: Dedicated studies evaluating effect of hepatic impairment on the pharmacokinetics of glycopyrrolate and formoterol were not conducted.
Patients with Renal Impairment: Dedicated studies evaluating effect of renal impairment on the pharmacokinetics of glycopyrrolate and formoterol were not conducted. When glycopyrrolate was administered IV in uremic patients undergoing renal transplantation, mean elimination half-life was significantly longer (46.8 minutes) than in healthy patients (18.6 minutes). The mean AUC (10.6 hr-mcg/L), mean plasma clearance (0.43 L/hr/kg), and mean 3-hour urine excretion (0.7%) for glycopyrrolate were also significantly different than those of controls (3.73 hr-mcg/L, 1.14 L/hr/kg, and 50%, respectively). A population pharmacokinetic analysis using BEVESPI AEROSPHERE showed that formoterol systemic exposure (AUC0-12) in subjects with COPD with moderate renal impairment (45 mL/min creatinine clearance) is expected to be approximately 45% higher compared to subjects with COPD with normal renal function (94 mL/min creatinine clearance).
Drug Interaction Studies
No pharmacokinetic interaction is expected when glycopyrrolate and formoterol fumarate are administered in combination by the inhaled route. Specific drug-drug interaction studies have not been performed with glycopyrrolate or formoterol fumarate.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
BEVESPI AEROSPHERE: No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with BEVESPI AEROSPHERE; however, separate studies of glycopyrrolate and formoterol fumarate are described below.
Glycopyrrolate
Long-term studies were conducted in mice using inhalation administration and rats using oral administration to evaluate the carcinogenic potential of glycopyrrolate.
In a 24-month inhalation carcinogenicity study in B6C3F1 mice, glycopyrrolate produced no evidence of tumorigenicity when administered to males or females at doses up to 705 and 335 mcg/kg/day, respectively (approximately 95 and 45 times the MRHDID of glycopyrrolate on a mcg/m2 basis, respectively).
In a 24-month carcinogenicity study in rats, glycopyrrolate produced no evidence of tumorigenicity when administered to males or females by oral gavage at dosages up to 40,000 mcg/kg/day (approximately 11,000 times the MRHDID of glycopyrrolate on a mcg/m2 basis).
Glycopyrrolate was not mutagenic or clastogenic in the Ames Salmonella/microsome plate test, in vitro mammalian cell micronucleus assay in TK6 cells or the in vivo micronucleus assay in rats.
Fertility and reproductive performance indices were unaffected in male and female rats that received glycopyrrolate by the subcutaneous route at doses up to 10,000 mcg/kg/day (approximately 2700 times the MRHDID on a mcg/m2 basis).
Formoterol Fumarate
Long-term studies were conducted in mice using oral administration and rats using inhalation administration to evaluate the carcinogenic potential of formoterol fumarate.
In a 24-month carcinogenicity study in CD-1 mice, formoterol fumarate at oral doses of 100 mcg/kg and above (approximately 25 times the MRHDID on a mcg/m2 basis) caused a dose-related increase in the incidence of uterine leiomyomas.
In a 24-month carcinogenicity study in Sprague-Dawley rats, an increased incidence of mesovarian leiomyoma and uterine leiomyosarcoma were observed at the inhaled dose of 130 mcg/kg (approximately 65 times the MRHDID on a mcg/m2 basis). No tumors were seen at 22 mcg/kg (approximately 10 times the MRHDID on a mcg/m2 basis).
Other beta-agonist drugs have similarly demonstrated increases in leiomyomas of the genital tract in female rodents. The relevance of these findings to human use is unknown.
Formoterol fumarate was not mutagenic or clastogenic in the Ames Salmonella/microsome plate test, mouse lymphoma test, chromosome aberration test in human lymphocytes, and rat micronucleus test.
A reduction in fertility and/or reproductive performance was identified in male rats treated with formoterol at an oral dose of 15,000 mcg/kg, (approximately 1900 times the MRHDID on an AUC basis). No such effect was seen at 3,000 mcg/kg (approximately 1500 times the MRHDID on a mcg/m2 basis). In a separate study with male rats treated with an oral dose of 15,000 mcg/kg (approximately 8000 times the MRHDID on a mcg/m2 basis), there were findings of testicular tubular atrophy and spermatic debris in the testes and oligospermia in the epididymides. No effect on fertility was detected in female rats at doses up to 15,000 mcg/kg (approximately 1000 times the MRHDID on an AUC basis).
-
14 CLINICAL STUDIES
The safety and efficacy of BEVESPI AEROSPHERE was evaluated in a clinical development program that included 8 dose-ranging trials and two placebo-controlled lung function trials of 24-weeks duration that included a 28-week extension study to evaluate safety over 1 year. The efficacy of BEVESPI AEROSPHERE is based on the dose ranging trials in 822 subjects with COPD and the 2 placebo-controlled confirmatory trials in 3,705 subjects with COPD.
14.1 Dose-Ranging Trials
Dose selection for BEVESPI AEROSPHERE for COPD was primarily based on data for the individual components, glycopyrrolate and formoterol fumarate, in COPD patients. Based on the findings from these studies, glycopyrrolate/formoterol fumarate 18/9.6 mcg administered twice-daily was evaluated in the confirmatory COPD trials.
Glycopyrrolate
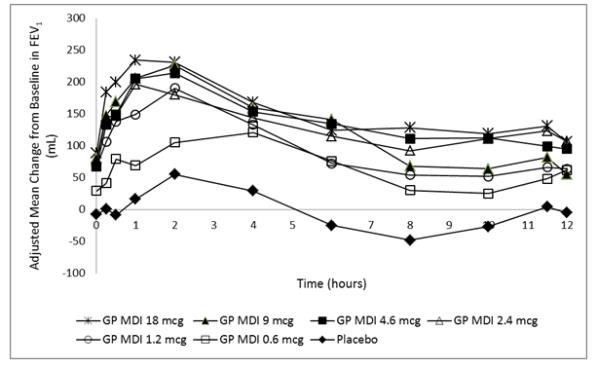
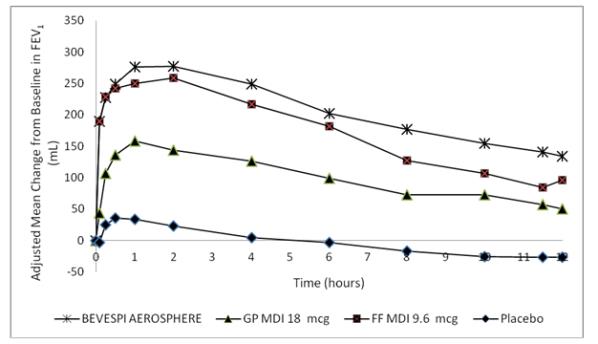
Dose selection for glycopyrrolate was supported by a 14-day, randomized, double-blind, placebo-controlled, incomplete-block crossover trial evaluating 6 doses of glycopyrrolate (GP MDI 18 to 0.6 mcg) administered twice daily and an open-label active control in 140 subjects with COPD. A dose ordering was observed, with the glycopyrrolate 18 mcg demonstrating larger improvements in FEV1 over 12 hours compared with glycopyrrolate 9, 4.6, 2.4, 1.2, and 0.6 mcg (Figure 1).
Figure 1 - Mean Change from Baseline in FEV1 over Time on Day 14 (MITT Population)
The difference from placebo in change from baseline in trough FEV1 after 14 days for the 18, 9, 4.6, 2.4, 1.2, and 0.6 mcg doses were 97 mL (95% CI: 45, 149), 88 mL (95% CI: 37, 139), 75 mL (95% CI: 24, 125), 84 mL (95% CI: 33, 135), 76 mL (95% CI: 22, 129), and 37 mL (95% CI: -17, 91), respectively. Two additional dose ranging trials (single-dose and 7-day trials) in subjects with COPD demonstrated minimal additional benefit at doses above 18 mcg of glycopyrrolate. The results supported the selection of 18 mcg of glycopyrrolate twice daily in the confirmatory COPD trials.
Evaluations of the appropriate dosing interval for glycopyrrolate were conducted by comparing to open-label ipratropium bromide inhalation aerosol administered four times daily. The results supported the selection of a twice-daily dosing interval for further evaluation in the confirmatory COPD trials.
Formoterol Fumarate
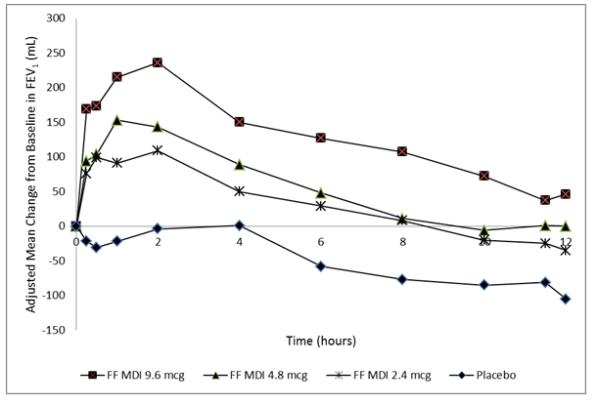
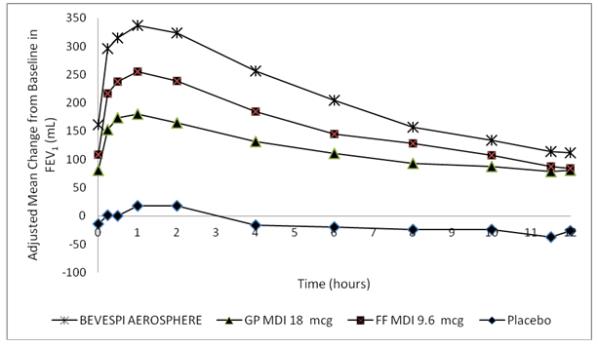
Dose selection for formoterol fumarate was supported by a single-dose, randomized, double-blind, placebo-controlled, crossover trial evaluating 3 doses of formoterol fumarate (FF MDI 9.6, 4.8, and 2.4 mcg), an open-label active control, and placebo in 34 subjects with COPD. A dose ordering was observed with the formoterol fumarate 9.6 mcg dose demonstrating larger improvements in FEV1 over 12 hours compared with the lower doses of 4.8 and 2.4 mcg (Figure 2).
Figure 2 - Mean Change from Baseline in FEV1 over Time on Day 1
The differences in mean change from baseline in normalized FEV1 AUC0-12 for formoterol fumarate 9.6, 4.8, and 2.4 mcg compared to placebo were 176 mL (95% CI: 138, 214), 103 (95% CI: 66, 140), and 81 (95% CI: 45, 118), respectively. These results provided support for the selection of 9.6 mcg of formoterol fumarate twice daily in the confirmatory COPD trials.
14.2 Confirmatory Trials
The clinical development program for BEVESPI AEROSPHERE included two (Trial 1 and Trial 2) 24-week, randomized, double-blind, placebo-controlled, parallel-group trials in subjects with moderate to very severe COPD designed to evaluate the efficacy of BEVESPI AEROSPHERE on lung function. The 24-week trials included 3,699 subjects that had a clinical diagnosis of COPD, were between 40 and 80 years of age, had a history of smoking greater than or equal to 10 pack-years, had a post-albuterol FEV1 less than 80% of predicted normal values, and had a ratio of FEV1/FVC of less than 0.7. The majority of patients were male (56%) and Caucasian (91%) with a mean age of 63 years and an average smoking history of 51 pack-years (54% current smokers). During screening, mean post-bronchodilator percent predicted FEV1 was 51% (range: 19% to 82%) and mean percent reversibility was 20% (range: -32% to 135%).
Trial 1 and Trial 2 evaluated BEVESPI AEROSPHERE (glycopyrrolate/formoterol fumarate) 18 mcg/9.6 mcg, glycopyrrolate 18 mcg, formoterol fumarate 9.6 mcg, and placebo administered twice daily (BID). Trial 1 also included an open-label active control. The primary endpoint was change from baseline in trough FEV1 at Week 24 compared with placebo, glycopyrrolate 18 mcg BID, and formoterol fumarate 9.6 mcg BID. The comparison of BEVESPI AEROSPHERE with glycopyrrolate 18 mcg and formoterol fumarate 9.6 mcg was assessed to evaluate the contribution of the individual components to BEVESPI AEROSPHERE. In both trials, BEVESPI AEROSPHERE demonstrated a larger increase in mean change from baseline in trough FEV1 at Week 24 relative to placebo, glycopyrrolate 18 mcg, and formoterol fumarate 9.6 mcg (Table 2).
Table 2 – Least Square (LS) Mean Change from Baseline in Morning Pre-dose Trough
FEV1 (mL) at Week 24 in Trial 1 and Trial 2 (Intent-to-Treat Population)- *
- The placebo, glycopyrrolate and formoterol fumarate comparators used the same inhaler and excipients as BEVESPI AEROSPHERE.
Trough FEV1 (mL) at Week 24
Difference from
Treatment
N
Placebo*
LS Mean
(95% CI)Glycopyrrolate
18 mcg BID*
LS Mean
(95% CI)Formoterol Fumarate
9.6 mcg BID*
LS Mean
(95% CI)Trial 1
BEVESPI AEROSPHERE
429
N=161
150 mL
(114, 186)N=344
59 mL
(31, 88)N=367
64 mL
(36, 92)Trial 2
BEVESPI
AEROSPHERE433
N=170
103 mL
(67, 140)N=367
54 mL
(25, 83)N=350
56 mL
(27, 85)N = Number in the intent to treat population
With the limited data available, there were consistent improvements in trough FEV1 with respect to age, sex, degree of airflow limitation, GOLD stage, smoking status, or inhaled corticosteroid use.
In Trials 1 and 2, serial spirometric evaluations were performed throughout the 12-hour dosing interval in a subset of subjects (n=718 and n=585, respectively) at Day 1 and Week 12. Results from Trial 1 are shown in Figure 3. In Trial 2, the results for BEVESPI AEROSPHERE in FEV1 AUC0-12h were similar to those observed in Trial 1.
Figure 3 - Mean Change from Baseline in FEV1 over Time at Day 1 and Week 12 (Trial 1)
Day 1
Week 12
In both trials, peak FEV1 was defined as the maximum FEV1 recorded within 2 hours after the dose of trial medication. The mean peak FEV1 improvement from baseline with BEVESPI AEROSPHERE compared with placebo at Week 24 was 291 mL (95% CI: 252, 331) and 267 mL (95% CI: 226, 308) in Trial 1 and Trial 2, respectively. BEVESPI AEROSPHERE demonstrated an onset of bronchodilatory treatment effect at 5 minutes after the first dose based on a mean increase in FEV1 compared to placebo of 187 mL (95% CI: 168, 205) and 186 mL (95% CI: 164, 207) in Trial 1 and Trial 2, respectively. In both Trial 1 and 2, subjects treated with BEVESPI AEROSPHERE used less daily rescue albuterol compared to subjects treated with placebo.
The St. George’s Respiratory Questionnaire (SGRQ) was assessed in Trials 1 and 2. In Trial 1, the SGRQ responder rate (defined as an improvement in score of 4 or more as threshold) was 37%, 30%, 35%, and 28% for BEVESPI AEROSPHERE, glycopyrrolate, formoterol fumarate, and placebo, respectively, with odds ratios of 1.4 (95% CI: 1.1, 1.8), 1.1 (95% CI: 0.9, 1.5), and 1.5 (95% CI: 1.1, 2.1), for BEVESPI AEROSPHERE vs. glycopyrrolate, BEVESPI AEROSPHERE vs. formoterol fumarate, and BEVESPI AEROSPHERE vs. placebo, respectively. In Trial 2, the trends were similar, with odds ratios of 1.2 (95% CI: 0.9, 1.6), 1.3 (95% CI: 1.0, 1.7), and 1.3 (95% CI: 0.9, 1.8), for BEVESPI AEROSPHERE vs. glycopyrrolate, BEVESPI AEROSPHERE vs. formoterol fumarate, and BEVESPI AEROSPHERE vs. placebo, respectively.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
BEVESPI AEROSPHERE Inhalation Aerosol:
- •
- 9 mcg glycopyrrolate and 4.8 mcg formoterol fumarate per inhalation
- •
- is supplied as a pressurized aluminum canister with an attached dose indicator, a white plastic actuator and mouthpiece, and an orange dust cap
- •
- each 120 inhalation canister has a net fill weight of 10.7 grams (NDC 0310-4600-12)
- •
- each canister is packaged in a foil pouch with desiccant sachet and is placed into a carton
- •
- each carton contains one canister and a Patient Information leaflet
The BEVESPI AEROSPHERE canister should only be used with the BEVESPI AEROSPHERE actuator, and the BEVESPI AEROSPHERE actuator should not be used with any other inhalation drug product.
The correct amount of medication in each inhalation cannot be assured after the label number of inhalations from the canister have been used, when the dose indicator display window shows zero, even though the canister may not feel completely empty. BEVESPI AEROSPHERE should be discarded when the dose indicator display window shows zero or 3 months after removal from the foil pouch, whichever comes first. Never immerse the canister into water to determine the amount remaining in the canister (“float test”).
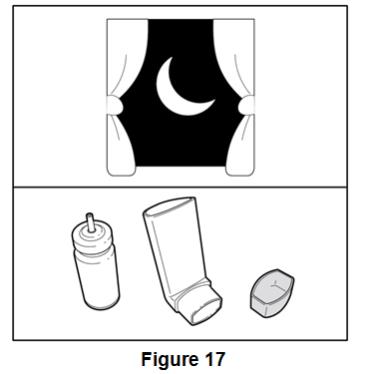
Store at controlled room temperature 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP].
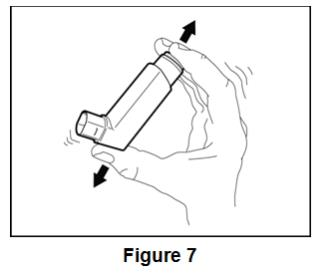
For best results, the canister should be at room temperature before use. Shake well before using. Keep out of reach of children.
CONTENTS UNDER PRESSURE
Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 49°C (120°F) may cause bursting. Never throw canister into fire or incinerator. Avoid spraying in eyes.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
Serious Asthma-Related Events: Inform patients that LABAs, such as formoterol fumarate, one of the active ingredients in BEVESPI AEROSPHERE, when used alone (without an inhaled corticosteroid), increase the risk of serious asthma-related events, including asthma-related death. BEVESPI AEROSPHERE is not indicated for the treatment of asthma [see Warnings and Precautions (5.1)].
Not for Acute Symptoms: Inform patients that BEVESPI AEROSPHERE is not meant to relieve acute symptoms of COPD and extra doses should not be used for that purpose. Advise them to treat acute symptoms with a rescue inhaler such as albuterol. Provide patients with such medicine and instruct them in how it should be used [see Warnings and Precautions (5.2)].
Instruct patients to seek medical attention immediately if they experience any of the following:
- •
- Symptoms get worse
- •
- Need for more inhalations than usual of their rescue inhaler
Patients should not stop therapy with BEVESPI AEROSPHERE without physician/provider guidance since symptoms may recur after discontinuation.
Do Not Use Additional Long-Acting Beta2-Agonists: Instruct patients to not use other medicines containing a LABA. Patients should not use more than the recommended dose of BEVESPI AEROSPHERE [see Warnings and Precautions (5.3)].
Instruct patients who have been taking inhaled, short-acting beta2-agonists on a regular basis to discontinue the regular use of these products and use them only for the symptomatic relief of acute symptoms.
Paradoxical Bronchospasm: As with other inhaled medicines, BEVESPI AEROSPHERE can cause paradoxical bronchospasm. If paradoxical bronchospasm occurs, instruct patients to discontinue BEVESPI AEROSPHERE [see Warnings and Precautions (5.4)].
Risks Associated With Beta2-Agonist Therapy: Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness. Instruct patients to consult a physician immediately should any of these signs or symptoms develop [see Warnings and Precautions (5.6)].
Worsening of Narrow Angle Glaucoma: Instruct patients to be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos, or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately should any of these signs or symptoms develop [see Warnings and Precautions (5.7)].
Worsening of Urinary Retention: Instruct patients to be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination). Instruct patients to consult a physician immediately should any of these signs or symptoms develop [see Warnings and Precautions (5.8)].
Instructions for Administering BEVESPI AEROSPHERE
It is important for patients to understand how to correctly administer BEVESPI AEROSPHERE [see Instructions for Use].
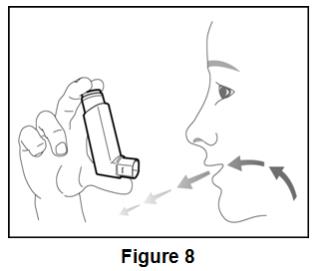
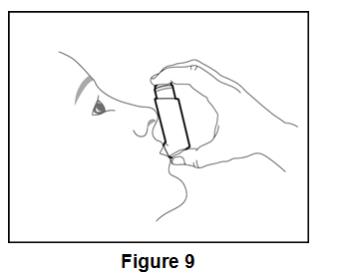
Inform patients to use 2 inhalations of BEVESPI AEROSPHERE orally twice daily (2 inhalations in the morning and 2 inhalations in the evening).
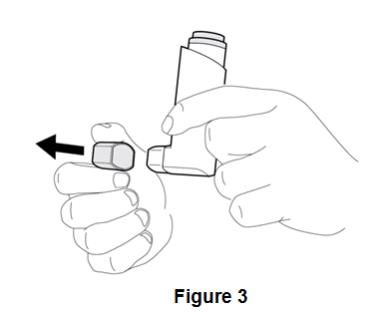
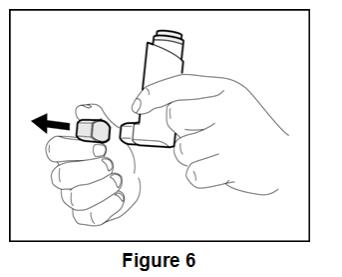
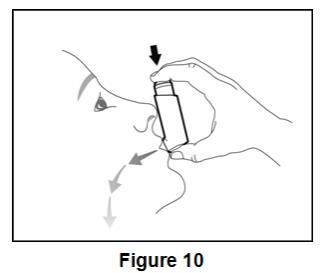
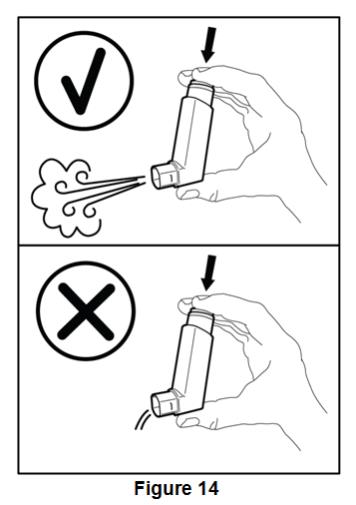
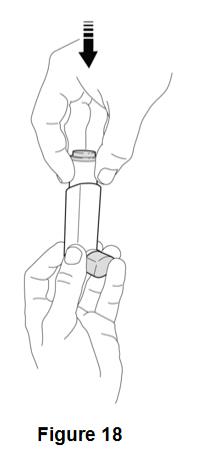
Instruct patients to prime BEVESPI AEROSPHERE before using it for the first time. Instruct patients to prime BEVESPI AEROSPHERE by releasing 4 sprays into the air away from their face, shaking well before each spray. Inform patients that BEVESPI AEROSPHERE must be re-primed when the inhaler has not been used for more than 7 days. Instruct patients to re-prime BEVESPI AEROSPHERE by releasing 2 sprays into the air away from their face, shaking well before each spray.
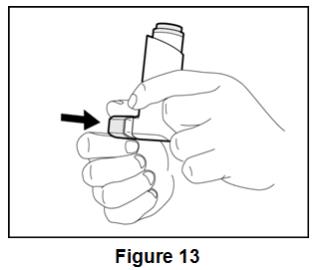
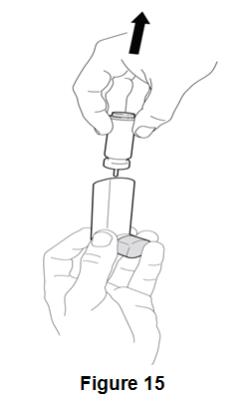
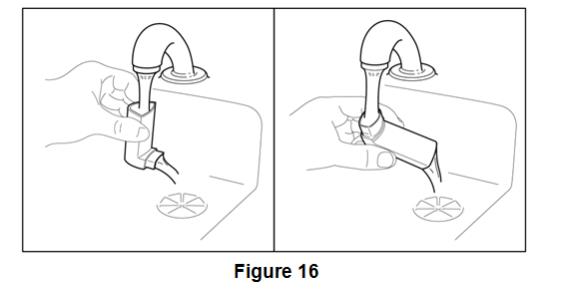
Inform patients that it is very important to clean BEVESPI AEROSPHERE 1 time each week so that medicine will not build up and block the spray through the mouthpiece [see Instructions for Use]. Instruct patients to clean BEVESPI AEROSPHERE by taking the canister out of the actuator, running warm water through the actuator, and allowing the actuator to air-dry overnight. Instruct patients to insert the canister back into the actuator after it is dry, and to re-prime BEVESPI AEROSPHERE. Instruct patients to re-prime BEVESPI AEROSPHERE by releasing 2 sprays into the air away from their face, shaking well before each spray.
Inform patients that if they miss a dose of BEVESPI AEROSPHERE, they should take their next dose at the usual time. Instruct patients to not use BEVESPI AEROSPHERE more often or more puffs than they have been prescribed.
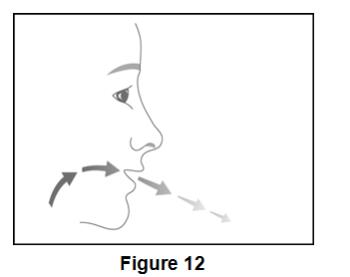
Instruct patients not to spray BEVESPI AEROSPHERE in their eyes. Inform patients that if they accidentally get BEVESPI AEROSPHERE in their eyes, to rinse their eyes with water, and if redness or irritation persists, to consult their healthcare provider.
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
BEVESPI, AEROSPHERE and BEVESPI AEROSPHERE are registered trademarks of the AstraZeneca group of companies.
©AstraZeneca 2019
-
PATIENT INFORMATION
BEVESPI AEROSPHERE®
(be-VES-pee AIR-oh-sfeer)
(glycopyrrolate and formoterol fumarate)
inhalation aerosol, for oral inhalation useWhat is BEVESPI AEROSPHERE?
BEVESPI AEROSPHERE combines an anticholinergic, glycopyrrolate, and a long-acting beta2-adrenergic agonist (LABA) medicine, formoterol fumarate.- •
- Anticholinergic and LABA medicines help the muscles around the airways in your lungs stay relaxed to prevent symptoms such as wheezing, cough, chest tightness, and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe.
- •
- BEVESPI AEROSPHERE is a prescription medicine used to treat COPD. COPD is a chronic lung disease that includes chronic bronchitis, emphysema, or both.
- •
- BEVESPI AEROSPHERE is used long term as 2 inhalations, 2 times each day in the morning and in the evening, to improve symptoms of COPD for better breathing.
- •
- BEVESPI AEROSPHERE is not for use to treat sudden symptoms of COPD. Always have a rescue inhaler (an inhaled, short-acting bronchodilator) with you to treat sudden symptoms. If you do not have a rescue inhaler, contact your healthcare provider to have one prescribed for you.
- •
- BEVESPI AEROSPHERE is not for the treatment of asthma. It is not known if BEVESPI AEROSPHERE is safe and effective in people with asthma.
- •
- BEVESPI AEROSPHERE should not be used in children. It is not known if BEVESPI AEROSPHERE is safe and effective in children.
Do not use BEVESPI AEROSPHERE if you:
- •
- are allergic to glycopyrrolate, formoterol fumarate, or to any of the ingredients in BEVESPI AEROSPHERE. See the end of this Patient Information leaflet for a complete list of ingredients.
- •
- have asthma.
Before using BEVESPI AEROSPHERE, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have heart problems
- •
- have high blood pressure
- •
- have seizures
- •
- have thyroid problems
- •
- have diabetes
- •
- have liver problems
- •
- have eye problems such as glaucoma. BEVESPI AEROSPHERE may make your glaucoma worse.
- •
- have prostate or bladder problems, or problems passing urine. BEVESPI AEROSPHERE may make these problems worse.
- •
- are allergic to any other medicines or food products
- •
- have any other medical conditions
- •
- are pregnant or plan to become pregnant. It is not known if BEVESPI AEROSPHERE may harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if the medicines, glycopyrrolate and formoterol fumarate, in BEVESPI AEROSPHERE pass into your breast milk and if they can harm your baby.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. BEVESPI AEROSPHERE and certain other medicines may interact with each other. This may cause serious side effects.
Especially tell your healthcare provider if you take:- •
- anticholinergics (including tiotropium, ipratropium, aclidinium, and umeclidinium)
- •
- other LABAs (including salmeterol, arformoterol, vilanterol, olodaterol, and indacaterol)
- •
- atropine
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine.
How should I use BEVESPI AEROSPHERE?
Read the step-by-step instructions for using BEVESPI AEROSPHERE at the end of this Patient Information.- •
- Do not use BEVESPI AEROSPHERE unless your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly.
- •
- Use BEVESPI AEROSPHERE exactly as prescribed. Do not use BEVESPI AEROSPHERE more often than prescribed.
- •
- Use 2 inhalations of BEVESPI AEROSPHERE, 2 times each day (in the morning and in the evening).
- •
- If you miss a dose of BEVESPI AEROSPHERE, take your next dose at the same time you normally do. Do not take more than your prescribed dose of BEVESPI AEROSPHERE.
- •
- If you take too much BEVESPI AEROSPHERE, call your healthcare provider or go to the nearest hospital emergency room right away if you have unusual symptoms, such as worsening shortness of breath, chest pain, increased heart rate, or shakiness.
- •
- Do not spray BEVESPI AEROSPHERE in your eyes. If BEVESPI AEROSPHERE gets in your eyes, rinse them well with water. If redness continues, call your healthcare provider.
- •
- Do not stop using BEVESPI AEROSPHERE unless told to do so by your healthcare provider because your symptoms might come back. Your healthcare provider will change your medicines as needed.
- •
- Do not use other medicines that contain a LABA or an anticholinergic for any reason. Ask your healthcare provider or pharmacist if any of your other medicines are LABA or anticholinergic containing medicines.
- •
- BEVESPI AEROSPHERE does not relieve sudden symptoms of COPD. Always have a rescue inhaler with you to treat sudden symptoms. If you do not have a rescue inhaler, call your healthcare provider to have one prescribed for you.
- •
- Call your healthcare provider or get medical care right away if your breathing problems get worse; you need to use your rescue inhaler more often than usual, or your rescue inhaler does not work as well to relieve your symptoms.
What are the possible side effects with BEVESPI AEROSPHERE?
BEVESPI AEROSPHERE can cause serious side effects, including:- •
- people with asthma who take LABA medicines, such as formoterol fumarate (one of the medicines in BEVESPI AEROSPHERE), without also using a medicine called an inhaled corticosteroid, have an increased risk of serious problems from asthma, including being hospitalized, needing a tube placed in their airway to help them breathe, or death.
- •
- Call your healthcare provider if breathing problems worsen over time while using BEVESPI AEROSPHERE. You may need a different treatment.
- •
-
Get emergency medical care if:
- ∘
- your breathing problems worsen quickly
- ∘
- you use your rescue inhaler medicine, but it does not relieve your breathing problems
- •
- using too much of a LABA medicine may cause:
-
- ∘
- chest pain
- ∘
- fast and irregular heartbeat
- ∘
- tremor
- ∘
- increased blood pressure
- ∘
- headache
- ∘
- nervousness
- •
- COPD symptoms can get worse over time. If your COPD symptoms worsen over time, do not increase your dose of BEVESPI AEROSPHERE, instead call your healthcare provider.
- •
- sudden breathing problems immediately after inhaling your medicine. If you have sudden breathing problems immediately after inhaling your medicine, stop using BEVESPI AEROSPHERE and call your healthcare provider right away.
- •
- serious allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following symptoms of a serious allergic reaction:
-
- ∘
- rash
- ∘
- hives
- ∘
- swelling of the face, mouth, and tongue
- ∘
- breathing problems
- •
- effects on your heart:
-
- ∘
- increase blood pressure
- ∘
- a fast or irregular heartbeat
- ∘
- chest pain
- •
- effects on your nervous system:
-
- ∘
- tremor
- ∘
- nervousness
- •
- changes in laboratory blood levels, including high levels of blood sugar (hyperglycemia) and low levels of potassium (hypokalemia) which may cause symptoms of muscle spasm, muscle weakness or abnormal heart rhythm.
- •
- new or worsened eye problems including acute narrow-angle glaucoma. Acute narrow-angle glaucoma can cause permanent loss of vision if not treated. Symptoms of acute narrow-angle glaucoma may include:
-
- ∘
- eye pain or discomfort
- ∘
- nausea or vomiting
- ∘
- blurred vision
- ∘
- seeing halos or bright colors around lights
- ∘
- red eyes
- If you have these symptoms, call your healthcare provider right away before taking another dose.
- •
- urinary retention. People who take BEVESPI AEROSPHERE may develop new or worse urinary retention. Symptoms of urinary retention may include:
-
- ∘
- difficulty urinating
- ∘
- painful urination
- ∘
- urinating frequently
- ∘
- urination in a weak stream or drips
- If you have these symptoms of urinary retention, stop taking BEVESPI AEROSPHERE and call your healthcare provider right away before taking another dose.
Common side effects of BEVESPI AEROSPHERE include: urinary tract infection and cough.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of BEVESPI AEROSPHERE. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to AstraZeneca at 1-800-236-9933.
How should I store BEVESPI AEROSPHERE?
- •
- Store BEVESPI AEROSPHERE at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- •
- Do not put a hole in the BEVESPI AEROSPHERE canister.
- •
- Do not use or store BEVESPI AEROSPHERE near heat or a flame. Temperatures above 120ºF (49ºC) may cause the canister to burst.
- •
- Do not throw the BEVESPI AEROSPHERE canister into a fire or an incinerator.
- •
- Throw away BEVESPI AEROSPHERE 3 months after you open the foil pouch or when the dose indicator reaches zero “0”, whichever comes first.
- •
- Keep BEVESPI AEROSPHERE and all medicines out of the reach of children.
General Information about the safe and effective use of BEVESPI AEROSPHERE
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use BEVESPI AEROSPHERE for a condition for which it was not prescribed. Do not give your BEVESPI AEROSPHERE to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about BEVESPI AEROSPHERE that is written for health professionals.Active ingredients: micronized glycopyrrolate and micronized formoterol fumarate
Inactive ingredients: hydrofluoroalkane (HFA 134a) and porous particles (comprised of DSPC [1,2-Distearoyl-sn-glycero-3-phosphocholine] and calcium chloride)
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850© AstraZeneca 2019
For more information, call 1-800-236-9933 or go to www.BEVESPI.com.
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: March 2023
- PRINCIPAL DISPLAY PANEL – 9 mcg/4.8 mcg per inhalation
-
INGREDIENTS AND APPEARANCE
BEVESPI AEROSPHERE
glycopyrrolate and formoterol fumarate aerosol, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0310-4600 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCOPYRROLATE (UNII: V92SO9WP2I) (GLYCOPYRRONIUM - UNII:A14FB57V1D) GLYCOPYRROLATE 9 ug FORMOTEROL FUMARATE (UNII: W34SHF8J2K) (FORMOTEROL - UNII:5ZZ84GCW8B) FORMOTEROL FUMARATE 4.8 ug Inactive Ingredients Ingredient Name Strength 1,2-DISTEAROYL-SN-GLYCERO-3-PHOSPHOCHOLINE (UNII: 043IPI2M0K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) NORFLURANE (UNII: DH9E53K1Y8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0310-4600-12 120 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 10/03/2016 2 NDC:0310-4600-28 28 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 05/01/2017 06/30/2021 3 NDC:0310-4600-39 28 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 08/01/2017 12/31/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208294 10/03/2016 Labeler - AstraZeneca Pharmaceuticals LP (054743190) Registrant - AstraZeneca PLC (230790719)