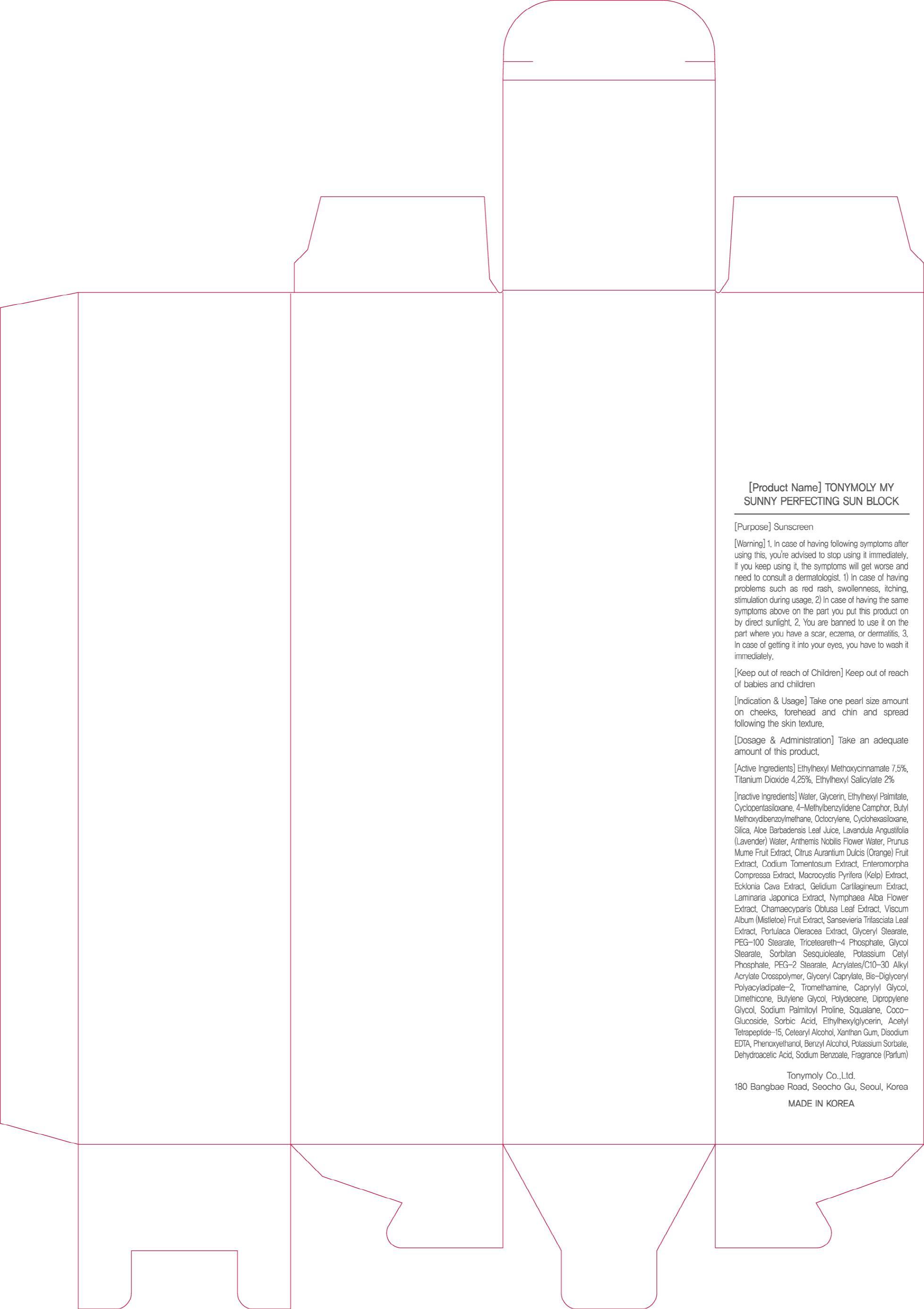
Label: TONYMOLY MY SUNNY PERFECTING SUN BLOCK- octinoxate, titanium dioxide, octisalate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 59078-047-01 - Packager: TONYMOLY CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 26, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive Ingredients:
Water, Glycerin, Ethylhexyl Palmitate, Cyclopentasiloxane, 4-Methylbenzylidene Camphor, Butyl Methoxydibenzoylmethane, Octocrylene, Cyclohexasiloxane, Silica, Aloe Barbadensis Leaf Juice, Lavandula Angustifolia (Lavender) Water, Anthemis Nobilis Flower Water, Prunus Mume Fruit Extract, Citrus Aurantium Dulcis (Orange) Fruit Extract, Codium Tomentosum Extract, Enteromorpha Compressa Extract, Macrocystis Pyrifera (Kelp) Extract, Ecklonia Cava Extract, Gelidium Cartilagineum Extract, Laminaria Japonica Extract, Nymphaea Alba Flower Extract, Chamaecyparis Obtusa Leaf Extract, Viscum Album (Mistletoe) Fruit Extract, Sansevieria Trifasciata Leaf Extract, Portulaca Oleracea Extract, Glyceryl Stearate, PEG-100 Stearate, Triceteareth-4 Phosphate, Glycol Stearate, Sorbitan Sesquioleate, Potassium Cetyl Phosphate, PEG-2 Stearate, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Glyceryl Caprylate, Bis-Diglyceryl Polyacyladipate-2, Tromethamine, Caprylyl Glycol, Dimethicone, Butylene Glycol, Polydecene, Dipropylene Glycol, Sodium Palmitoyl Proline, Squalane, Coco-Glucoside, Sorbic Acid, Ethylhexylglycerin, Acetyl Tetrapeptide-15, Cetearyl Alcohol, Xanthan Gum, Disodium EDTA, Phenoxyethanol, Benzyl Alcohol, Potassium Sorbate, Dehydroacetic Acid, Sodium Benzoate, Fragrance(Parfum) - PURPOSE
-
WARNINGS
Warning:
1. In case of having following symptoms after using this, you're advised to stop using it immediately. If you keep using it, the symptoms will get worse and need to consult a dermatologist.
1) In case of having problems such as red rash, swollenness, itching, stimulation during usage.
2) In case of having the same symptoms above on the part you put this product on by direct sunlight.
2. You are banned to use it on the part where you have a scar, eczema, or dermatitis.
3. In case of getting it into your eyes, you have to wash it immediately. - KEEP OUT OF REACH OF CHILDREN
- INDICATIONS AND USAGE
- DOSAGE AND ADMINISTRATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TONYMOLY MY SUNNY PERFECTING SUN BLOCK
octinoxate, titanium dioxide, octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59078-047 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mg in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4.25 mg in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59078-047-01 100 mL in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/01/2014 Labeler - TONYMOLY CO., LTD. (688216798) Registrant - TONYMOLY CO., LTD. (688216798) Establishment Name Address ID/FEI Business Operations TONYMOLY CO., LTD. 688216798 manufacture(59078-047)