Label: SUSTIVA- efavirenz tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-4668-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0056-0510
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 2, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SUSTIVA safely and effectively. See full prescribing information for SUSTIVA.
SUSTIVA® (efavirenz) capsules for oral use
SUSTIVA® (efavirenz) tablets for oral use
Initial U.S. Approval: 1998RECENT MAJOR CHANGES
INDICATIONS AND USAGE
SUSTIVA is a non-nucleoside reverse transcriptase inhibitor indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 infection. (1)
DOSAGE AND ADMINISTRATION
- SUSTIVA should be taken orally once daily on an empty stomach, preferably at bedtime. (2)
- Recommended adult dose: 600 mg. (2.1)
- With voriconazole, increase voriconazole maintenance dose to 400 mg every 12 hours and decrease SUSTIVA dose to 300 mg once daily using the capsule formulation. (2.1)
- With rifampin, increase SUSTIVA dose to 800 mg once daily for patients weighing 50 kg or more. (2.1)
Pediatric Patients at Least 3 Years and at Least 10 kg (2.2) kg lbs dose kg lbs dose 10 - <15 22 - <33 200 mg 25 - <32.5 55 - <71.5 350 mg 15 - <20 33 - <44 250 mg 32.5 - <40 71.5 - <88 400 mg 20 - <25 44 - <55 300 mg at least 40 at least 88 600 mg CONTRAINDICATIONS
- SUSTIVA is contraindicated in patients with previously demonstrated hypersensitivity (eg, Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions) to any of the components of this product. (4.1)
- For some drugs, competition for CYP3A by efavirenz could result in inhibition of their metabolism and create the potential for serious and/or life-threatening adverse reactions (eg, cardiac arrhythmias, prolonged sedation, or respiratory depression). (4.2)
WARNINGS AND PRECAUTIONS
- Do not use as a single agent or add on as a sole agent to a failing regimen. Consider potential for cross resistance when choosing other agents. (5.2)
- Not recommended with ATRIPLA, which contains efavirenz, emtricitabine, and tenofovir disoproxil fumarate, unless needed for dose adjustment when coadministered with rifampin. (5.3)
- Serious psychiatric symptoms: Immediate medical evaluation is recommended for serious psychiatric symptoms such as severe depression or suicidal ideation. (5.4, 17.5)
- Nervous system symptoms (NSS): NSS are frequent, usually begin 1-2 days after initiating therapy and resolve in 2-4 weeks. Dosing at bedtime may improve tolerability. NSS are not predictive of onset of psychiatric symptoms. (5.5, 6.1, 17.4)
- Pregnancy: Fetal harm can occur when administered to a pregnant woman during the first trimester. Women should be apprised of the potential harm to the fetus. (5.6, 17.7) Pregnancy registry is available. (8.1)
- Hepatotoxicity: Monitor liver function tests before and during treatment in patients with underlying hepatic disease, including hepatitis B or C coinfection, marked transaminase elevations, or who are taking medications associated with liver toxicity. Among reported cases of hepatic failure, a few occurred in patients with no pre-existing hepatic disease. (5.8, 6.1, 8.6)
- Rash: Rash usually begins within 1-2 weeks after initiating therapy and resolves within 4 weeks. Discontinue if severe rash develops. (5.7, 6.1, 17.6)
- Convulsions: Use caution in patients with a history of seizures. (5.9)
- Lipids: Total cholesterol and triglyceride elevations. Monitor before therapy and periodically thereafter. (5.10)
- Immune reconstitution syndrome: May necessitate further evaluation and treatment. (5.11)
- Redistribution/accumulation of body fat: Observed in patients receiving antiretroviral therapy. (5.12, 17.8)
ADVERSE REACTIONS
Most common adverse reactions (>5%, moderate-severe) are rash, dizziness, nausea, headache, fatigue, insomnia, and vomiting. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb at 1-800-721-5072 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Coadministration of efavirenz can alter the concentrations of other drugs and other drugs may alter the concentrations of efavirenz. The potential for drug-drug interactions must be considered before and during therapy. (4.2, 7.1, 12.3)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Women should avoid pregnancy during SUSTIVA therapy and for 12 weeks after discontinuation. (5.6)
- Nursing mothers: Women infected with HIV should be instructed not to breast-feed. (8.3)
- Hepatic impairment: SUSTIVA is not recommended for patients with moderate or severe hepatic impairment. Use caution in patients with mild hepatic impairment. (8.6)
- Pediatric patients: The incidence of rash was higher than in adults. (5.7, 6.1, 6.2, 8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2013
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Adults
2.2 Pediatric Patients
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Contraindicated Drugs
5 WARNINGS AND PRECAUTIONS
5.1 Drug Interactions
5.2 Resistance
5.3 Coadministration with Related Products
5.4 Psychiatric Symptoms
5.5 Nervous System Symptoms
5.6 Reproductive Risk Potential
5.7 Rash
5.8 Hepatotoxicity
5.9 Convulsions
5.10 Lipid Elevations
5.11 Immune Reconstitution Syndrome
5.12 Fat Redistribution
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience in Adults
6.2 Clinical Trial Experience in Pediatric Patients
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
7.2 Cannabinoid Test Interaction
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Capsules
16.2 Tablets
16.3 Storage
17 PATIENT COUNSELING INFORMATION
17.1 Drug Interactions
17.2 General Information for Patients
17.3 Dosing Instructions
17.4 Nervous System Symptoms
17.5 Psychiatric Symptoms
17.6 Rash
17.7 Reproductive Risk Potential
17.8 Fat Redistribution
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
SUSTIVA® (efavirenz) in combination with other antiretroviral agents is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection. This indication is based on two clinical trials of at least one year duration that demonstrated prolonged suppression of HIV RNA [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Adults
The recommended dosage of SUSTIVA (efavirenz) is 600 mg orally, once daily, in combination with a protease inhibitor and/or nucleoside analogue reverse transcriptase inhibitors (NRTIs). It is recommended that SUSTIVA be taken on an empty stomach, preferably at bedtime. The increased efavirenz concentrations observed following administration of SUSTIVA with food may lead to an increase in frequency of adverse reactions [see Clinical Pharmacology (12.3)]. Dosing at bedtime may improve the tolerability of nervous system symptoms [see Warnings and Precautions (5.5), Adverse Reactions (6.1), and Patient Counseling Information (17.4)].
Concomitant Antiretroviral Therapy
SUSTIVA must be given in combination with other antiretroviral medications [see Indications and Usage (1), Warnings and Precautions (5.2), Drug Interactions (7.1), and Clinical Pharmacology (12.3)].
Dosage Adjustment
If SUSTIVA is coadministered with voriconazole, the voriconazole maintenance dose should be increased to 400 mg every 12 hours and the SUSTIVA dose should be decreased to 300 mg once daily using the capsule formulation (one 200-mg and two 50-mg capsules or six 50-mg capsules). SUSTIVA tablets should not be broken. See Drug Interactions (7.1, Table 7) and Clinical Pharmacology (12.3, Tables 8 and 9).
If SUSTIVA is coadministered with rifampin to patients weighing 50 kg or more, an increase in the dose of SUSTIVA to 800 mg once daily is recommended [see Drug Interactions (7.1, Table 7) and Clinical Pharmacology (12.3, Table 9)].
2.2 Pediatric Patients
It is recommended that SUSTIVA be taken on an empty stomach, preferably at bedtime. Table 1 describes the recommended dose of SUSTIVA for pediatric patients 3 years of age or older and weighing between 10 and 40 kg [see Use in Specific Populations (8.4)]. The recommended dosage of SUSTIVA for pediatric patients weighing greater than 40 kg is 600 mg once daily.
Table 1: Pediatric Dose to be Administered Once Daily Body Weight SUSTIVA Dose (mg) kg lbs 10 to less than 15 22 to less than 33 200 15 to less than 20 33 to less than 44 250 20 to less than 25 44 to less than 55 300 25 to less than 32.5 55 to less than 71.5 350 32.5 to less than 40 71.5 to less than 88 400 at least 40 at least 88 600 -
3 DOSAGE FORMS AND STRENGTHS
• Capsules
200-mg capsules are gold color, reverse printed with “SUSTIVA” on the body and imprinted “200 mg” on the cap.
50-mg capsules are gold color and white, printed with “SUSTIVA” on the gold color cap and reverse printed “50 mg” on the white body.
• Tablets
600-mg tablets are yellow, capsular-shaped, film-coated tablets, with “SUSTIVA” printed on both sides.
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
SUSTIVA is contraindicated in patients with previously demonstrated clinically significant hypersensitivity (eg, Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions) to any of the components of this product.
4.2 Contraindicated Drugs
For some drugs, competition for CYP3A by efavirenz could result in inhibition of their metabolism and create the potential for serious and/or life-threatening adverse reactions (eg, cardiac arrhythmias, prolonged sedation, or respiratory depression). Drugs that are contraindicated with SUSTIVA are listed in Table 2.
Table 2: Drugs That Are Contraindicated or Not Recommended for Use With SUSTIVA Drug Class: Drug Name Clinical Comment Antimigraine: ergot derivatives (dihydroergotamine, ergonovine, ergotamine, methylergonovine) Potential for serious and/or life-threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues. Benzodiazepines: midazolam, triazolam Potential for serious and/or life-threatening reactions such as prolonged or increased sedation or respiratory depression. Calcium channel blocker: bepridil Potential for serious and/or life-threatening reactions such as cardiac arrhythmias. GI motility agent: cisapride Potential for serious and/or life-threatening reactions such as cardiac arrhythmias. Neuroleptic: pimozide Potential for serious and/or life-threatening reactions such as cardiac arrhythmias. St. John’s wort (Hypericum perforatum) May lead to loss of virologic response and possible resistance to efavirenz or to the class of non-nucleoside reverse transcriptase inhibitors (NNRTIs). -
5 WARNINGS AND PRECAUTIONS
5.1 Drug Interactions
Efavirenz plasma concentrations may be altered by substrates, inhibitors, or inducers of CYP3A. Likewise, efavirenz may alter plasma concentrations of drugs metabolized by CYP3A or CYP2B6 [see Contraindications (4.2) and Drug Interactions (7.1)].
5.2 Resistance
SUSTIVA must not be used as a single agent to treat HIV-1 infection or added on as a sole agent to a failing regimen. Resistant virus emerges rapidly when efavirenz is administered as monotherapy. The choice of new antiretroviral agents to be used in combination with efavirenz should take into consideration the potential for viral cross-resistance.
5.3 Coadministration with Related Products
Coadministration of SUSTIVA with ATRIPLA (efavirenz 600 mg/emtricitabine 200 mg/tenofovir disoproxil fumarate 300 mg) is not recommended unless needed for dose adjustment (eg, with rifampin), since efavirenz is one of its active ingredients.
5.4 Psychiatric Symptoms
Serious psychiatric adverse experiences have been reported in patients treated with SUSTIVA. In controlled trials of 1008 patients treated with regimens containing SUSTIVA for a mean of 2.1 years and 635 patients treated with control regimens for a mean of 1.5 years, the frequency (regardless of causality) of specific serious psychiatric events among patients who received SUSTIVA or control regimens, respectively, were severe depression (2.4%, 0.9%), suicidal ideation (0.7%, 0.3%), nonfatal suicide attempts (0.5%, 0), aggressive behavior (0.4%, 0.5%), paranoid reactions (0.4%, 0.3%), and manic reactions (0.2%, 0.3%). When psychiatric symptoms similar to those noted above were combined and evaluated as a group in a multifactorial analysis of data from Study 006, treatment with efavirenz was associated with an increase in the occurrence of these selected psychiatric symptoms. Other factors associated with an increase in the occurrence of these psychiatric symptoms were history of injection drug use, psychiatric history, and receipt of psychiatric medication at study entry; similar associations were observed in both the SUSTIVA and control treatment groups. In Study 006, onset of new serious psychiatric symptoms occurred throughout the study for both SUSTIVA-treated and control-treated patients. One percent of SUSTIVA-treated patients discontinued or interrupted treatment because of one or more of these selected psychiatric symptoms. There have also been occasional postmarketing reports of death by suicide, delusions, and psychosis-like behavior, although a causal relationship to the use of SUSTIVA cannot be determined from these reports. Patients with serious psychiatric adverse experiences should seek immediate medical evaluation to assess the possibility that the symptoms may be related to the use of SUSTIVA, and if so, to determine whether the risks of continued therapy outweigh the benefits. See Adverse Reactions (6.1).
5.5 Nervous System Symptoms
Fifty-three percent (531/1008) of patients receiving SUSTIVA in controlled trials reported central nervous system symptoms (any grade, regardless of causality) compared to 25% (156/635) of patients receiving control regimens [see Adverse Reactions (6.1, Table 4)]. These symptoms included, but were not limited to, dizziness (28.1% of the 1008 patients), insomnia (16.3%), impaired concentration (8.3%), somnolence (7.0%), abnormal dreams (6.2%), and hallucinations (1.2%). These symptoms were severe in 2.0% of patients, and 2.1% of patients discontinued therapy as a result. These symptoms usually begin during the first or second day of therapy and generally resolve after the first 2-4 weeks of therapy. After 4 weeks of therapy, the prevalence of nervous system symptoms of at least moderate severity ranged from 5% to 9% in patients treated with regimens containing SUSTIVA and from 3% to 5% in patients treated with a control regimen. Patients should be informed that these common symptoms were likely to improve with continued therapy and were not predictive of subsequent onset of the less frequent psychiatric symptoms [see Warnings and Precautions (5.4)]. Dosing at bedtime may improve the tolerability of these nervous system symptoms [see Dosage and Administration (2)].
Analysis of long-term data from Study 006 (median follow-up 180 weeks, 102 weeks, and 76 weeks for patients treated with SUSTIVA + zidovudine + lamivudine, SUSTIVA + indinavir, and indinavir + zidovudine + lamivudine, respectively) showed that, beyond 24 weeks of therapy, the incidences of new-onset nervous system symptoms among SUSTIVA-treated patients were generally similar to those in the indinavir-containing control arm.
Patients receiving SUSTIVA should be alerted to the potential for additive central nervous system effects when SUSTIVA is used concomitantly with alcohol or psychoactive drugs.
Patients who experience central nervous system symptoms such as dizziness, impaired concentration, and/or drowsiness should avoid potentially hazardous tasks such as driving or operating machinery.
5.6 Reproductive Risk Potential
Pregnancy Category D. Efavirenz may cause fetal harm when administered during the first trimester to a pregnant woman. Pregnancy should be avoided in women receiving SUSTIVA. Barrier contraception must always be used in combination with other methods of contraception (eg, oral or other hormonal contraceptives). Because of the long half-life of efavirenz, use of adequate contraceptive measures for 12 weeks after discontinuation of SUSTIVA is recommended. Women of childbearing potential should undergo pregnancy testing before initiation of SUSTIVA. If this drug is used during the first trimester of pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential harm to the fetus.
There are no adequate and well-controlled studies in pregnant women. SUSTIVA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus, such as in pregnant women without other therapeutic options. [See Use in Specific Populations (8.1).]
5.7 Rash
In controlled clinical trials, 26% (266/1008) of patients treated with 600 mg SUSTIVA experienced new-onset skin rash compared with 17% (111/635) of patients treated in control groups [see Adverse Reactions (6.1, Table 5)]. Rash associated with blistering, moist desquamation, or ulceration occurred in 0.9% (9/1008) of patients treated with SUSTIVA. The incidence of Grade 4 rash (eg, erythema multiforme, Stevens-Johnson syndrome) in patients treated with SUSTIVA in all studies and expanded access was 0.1%. Rashes are usually mild-to-moderate maculopapular skin eruptions that occur within the first 2 weeks of initiating therapy with efavirenz (median time to onset of rash in adults was 11 days) and, in most patients continuing therapy with efavirenz, rash resolves within 1 month (median duration, 16 days). The discontinuation rate for rash in clinical trials was 1.7% (17/1008). SUSTIVA can be reinitiated in patients interrupting therapy because of rash. SUSTIVA should be discontinued in patients developing severe rash associated with blistering, desquamation, mucosal involvement, or fever. Appropriate antihistamines and/or corticosteroids may improve the tolerability and hasten the resolution of rash. For patients who have had a life-threatening cutaneous reaction (eg, Stevens-Johnson syndrome), alternative therapy should be considered [see also Contraindications (4.1)].
Rash was reported in 26 of 57 pediatric patients (46%) treated with SUSTIVA capsules [see Adverse Reactions (6.1, 6.2)]. One pediatric patient experienced Grade 3 rash (confluent rash with fever), and two patients had Grade 4 rash (erythema multiforme). The median time to onset of rash in pediatric patients was 8 days. Prophylaxis with appropriate antihistamines before initiating therapy with SUSTIVA in pediatric patients should be considered.
5.8 Hepatotoxicity
Monitoring of liver enzymes before and during treatment is recommended for patients with underlying hepatic disease, including hepatitis B or C infection; patients with marked transaminase elevations; and patients treated with other medications associated with liver toxicity [see Adverse Reactions (6.1) and Use in Specific Populations (8.6)]. A few of the postmarketing reports of hepatic failure occurred in patients with no pre-existing hepatic disease or other identifiable risk factors [see Adverse Reactions (6.3)]. Liver enzyme monitoring should also be considered for patients without pre-existing hepatic dysfunction or other risk factors. In patients with persistent elevations of serum transaminases to greater than five times the upper limit of the normal range, the benefit of continued therapy with SUSTIVA needs to be weighed against the unknown risks of significant liver toxicity.
5.9 Convulsions
Convulsions have been observed in patients receiving efavirenz, generally in the presence of known medical history of seizures [see Nonclinical Toxicology (13.2)]. Caution must be taken in any patient with a history of seizures. Patients who are receiving concomitant anticonvulsant medications primarily metabolized by the liver, such as phenytoin and phenobarbital, may require periodic monitoring of plasma levels [see Drug Interactions (7.1)].
5.10 Lipid Elevations
Treatment with SUSTIVA has resulted in increases in the concentration of total cholesterol and triglycerides [see Adverse Reactions (6.1)]. Cholesterol and triglyceride testing should be performed before initiating SUSTIVA therapy and at periodic intervals during therapy.
5.11 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including SUSTIVA. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.12 Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
-
6 ADVERSE REACTIONS
The most significant adverse reactions observed in patients treated with SUSTIVA are:
- psychiatric symptoms [see Warnings and Precautions (5.4)],
- nervous system symptoms [see Warnings and Precautions (5.5)],
- rash [see Warnings and Precautions (5.7)].
The most common (>5% in either efavirenz treatment group) adverse reactions of at least moderate severity among patients in Study 006 treated with SUSTIVA in combination with zidovudine/lamivudine or indinavir were rash, dizziness, nausea, headache, fatigue, insomnia, and vomiting.
6.1 Clinical Trials Experience in Adults
Because clinical studies are conducted under widely varying conditions, the adverse reaction rates reported cannot be directly compared to rates in other clinical studies and may not reflect the rates observed in clinical practice.
Selected clinical adverse reactions of moderate or severe intensity observed in ≥2% of SUSTIVA-treated patients in two controlled clinical trials are presented in Table 3.
Table 3: Selected Treatment-Emergenta Adverse Reactions of Moderate or Severe Intensity Reported in ≥2% of SUSTIVA-Treated Patients in Studies 006 and ACTG 364 Study 006
LAM-, NNRTI-, and Protease Inhibitor-Naive PatientsStudy ACTG 364
NRTI-experienced, NNRTI-, and Protease Inhibitor-Naive PatientsAdverse Reactions SUSTIVAb
+
ZDV/LAM
(n=412)SUSTIVAb
+
Indinavir
(n=415)Indinavir
+
ZDV/LAM
(n=401)SUSTIVAb
+ Nelfinavir
+ NRTIs
(n=64)SUSTIVAb
+
NRTIs
(n=65)Nelfinavir
+
NRTIs
(n=66)180 weeksc 102 weeksc 76 weeksc 71.1 weeksc 70.9 weeksc 62.7 weeksc a Includes adverse events at least possibly related to study drug or of unknown relationship for Study 006. Includes all adverse events regardless of relationship to study drug for Study ACTG 364. b SUSTIVA provided as 600 mg once daily. c Median duration of treatment. d Includes erythema multiforme, rash, rash erythematous, rash follicular, rash maculopapular, rash petechial, rash pustular, and urticaria for Study 006 and macules, papules, rash, erythema, redness, inflammation, allergic rash, urticaria, welts, hives, itchy, and pruritus for ACTG 364. — = Not Specified. ZDV = zidovudine, LAM = lamivudine. Body as a Whole Fatigue 8% 5% 9% 0 2% 3% Pain 1% 2% 8% 13% 6% 17% Central and Peripheral Nervous System Dizziness 9% 9% 2% 2% 6% 6% Headache 8% 5% 3% 5% 2% 3% Insomnia 7% 7% 2% 0 0 2% Concentration impaired 5% 3% <1% 0 0 0 Abnormal dreams 3% 1% 0 — — — Somnolence 2% 2% <1% 0 0 0 Anorexia 1% <1% <1% 0 2% 2% Gastrointestinal Nausea 10% 6% 24% 3% 2% 2% Vomiting 6% 3% 14% — — — Diarrhea 3% 5% 6% 14% 3% 9% Dyspepsia 4% 4% 6% 0 0 2% Abdominal pain 2% 2% 5% 3% 3% 3% Psychiatric Anxiety 2% 4% <1% — — — Depression 5% 4% <1% 3% 0 5% Nervousness 2% 2% 0 2% 0 2% Skin & Appendages Rashd 11% 16% 5% 9% 5% 9% Pruritus <1% 1% 1% 9% 5% 9% Pancreatitis has been reported, although a causal relationship with efavirenz has not been established. Asymptomatic increases in serum amylase levels were observed in a significantly higher number of patients treated with efavirenz 600 mg than in control patients (see Laboratory Abnormalities).
Nervous System Symptoms
For 1008 patients treated with regimens containing SUSTIVA and 635 patients treated with a control regimen in controlled trials, Table 4 lists the frequency of symptoms of different degrees of severity and gives the discontinuation rates for one or more of the following nervous system symptoms: dizziness, insomnia, impaired concentration, somnolence, abnormal dreaming, euphoria, confusion, agitation, amnesia, hallucinations, stupor, abnormal thinking, and depersonalization [see Warnings and Precautions (5.5)]. The frequencies of specific central and peripheral nervous system symptoms are provided in Table 3.
Table 4: Percent of Patients with One or More Selected Nervous System Symptomsa,b Percent of Patients with: SUSTIVA 600 mg Once Daily
(n=1008)Control Groups
(n=635)% % a Includes events reported regardless of causality. b Data from Study 006 and three Phase 2/3 studies. c “Mild” = Symptoms which do not interfere with patient’s daily activities. d “Moderate” = Symptoms which may interfere with daily activities. e “Severe” = Events which interrupt patient’s usual daily activities. Symptoms of any severity 52.7 24.6 Mild symptomsc 33.3 15.6 Moderate symptomsd 17.4 7.7 Severe symptomse 2.0 1.3 Treatment discontinuation as a result of symptoms 2.1 1.1 Psychiatric Symptoms
Serious psychiatric adverse experiences have been reported in patients treated with SUSTIVA. In controlled trials, psychiatric symptoms observed at a frequency of >2% among patients treated with SUSTIVA or control regimens, respectively, were depression (19%, 16%), anxiety (13%, 9%), and nervousness (7%, 2%).
Rash
For 1008 adult and 57 pediatric patients treated with regimens containing SUSTIVA and 635 patients treated with a control regimen in controlled trials, the frequency of rash by NCI grade and the discontinuation rates as a result of rash in clinical studies are provided in Table 5 [see Warnings and Precautions (5.7)].
Table 5: Percent of Patients with Treatment-Emergent Rasha,b Percent of Patients with: Description of Rash Gradec SUSTIVA 600 mg Once Daily Adults
(n=1008)SUSTIVA
Pediatric Patients
(n=57)Control Groups
Adults
(n=635)% % % a Includes events reported regardless of causality. b Data from Study 006 and three Phase 2/3 studies. c NCI Grading System. Rash of any grade — 26.3 45.6 17.5 Grade 1 rash Erythema, pruritus 10.7 8.8 9.8 Grade 2 rash Diffuse maculopapular rash, dry desquamation 14.7 31.6 7.4 Grade 3 rash Vesiculation, moist desquamation, ulceration 0.8 1.8 0.3 Grade 4 rash Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, necrosis requiring surgery, exfoliative dermatitis 0.1 3.5 0.0 Treatment discontinuation as a result of rash — 1.7 8.8 0.3 As seen in Table 5, rash is more common in pediatric patients and more often of higher grade (ie, more severe) [see Warnings and Precautions (5.7)].
Experience with SUSTIVA in patients who discontinued other antiretroviral agents of the NNRTI class is limited. Nineteen patients who discontinued nevirapine because of rash have been treated with SUSTIVA. Nine of these patients developed mild-to-moderate rash while receiving therapy with SUSTIVA, and two of these patients discontinued because of rash.
Laboratory Abnormalities
Selected Grade 3-4 laboratory abnormalities reported in ≥2% of SUSTIVA-treated patients in two clinical trials are presented in Table 6.
Table 6: Selected Grade 3-4 Laboratory Abnormalities Reported in ≥2% of SUSTIVA-Treated Patients in Studies 006 and ACTG 364 Study 006
LAM-, NNRTI-, and Protease
Inhibitor-Naive PatientsStudy ACTG 364
NRTI-experienced,
NNRTI-, and Protease
Inhibitor-Naive PatientsVariable Limit SUSTIVAa
+ ZDV/LAM
(n=412)SUSTIVAa
+ Indinavir
(n=415)Indinavir
+ ZDV/LAM
(n=401)SUSTIVAa
+ Nelfinavir
+ NRTIs
(n=64)SUSTIVAa
+ NRTIs
(n=65)Nelfinavir
+ NRTIs
(n=66)180 weeksb 102 weeksb 76 weeksb 71.1 weeksb 70.9 weeksb 62.7 weeksb a SUSTIVA provided as 600 mg once daily. b Median duration of treatment. c Isolated elevations of GGT in patients receiving SUSTIVA may reflect enzyme induction not associated with liver toxicity. d Nonfasting. ZDV = zidovudine, LAM = lamivudine, ULN = Upper limit of normal, ALT = alanine aminotransferase,
AST = aspartate aminotransferase, GGT = gamma-glutamyltransferase.Chemistry ALT >5 x ULN 5% 8% 5% 2% 6% 3% AST >5 x ULN 5% 6% 5% 6% 8% 8% GGTc >5 x ULN 8% 7% 3% 5% 0 5% Amylase >2 x ULN 4% 4% 1% 0 6% 2% Glucose >250 mg/dL 3% 3% 3% 5% 2% 3% Triglyceridesd ≥751 mg/dL 9% 6% 6% 11% 8% 17% Hematology Neutrophils <750/mm3 10% 3% 5% 2% 3% 2% Patients Coinfected with Hepatitis B or C
Liver function tests should be monitored in patients with a history of hepatitis B and/or C. In the long-term data set from Study 006, 137 patients treated with SUSTIVA-containing regimens (median duration of therapy, 68 weeks) and 84 treated with a control regimen (median duration, 56 weeks) were seropositive at screening for hepatitis B (surface antigen positive) and/or C (hepatitis C antibody positive). Among these coinfected patients, elevations in AST to greater than five times ULN developed in 13% of patients in the SUSTIVA arms and 7% of those in the control arm, and elevations in ALT to greater than five times ULN developed in 20% of patients in the SUSTIVA arms and 7% of patients in the control arm. Among coinfected patients, 3% of those treated with SUSTIVA-containing regimens and 2% in the control arm discontinued from the study because of liver or biliary system disorders [see Warnings and Precautions (5.8)].
Lipids
Increases from baseline in total cholesterol of 10-20% have been observed in some uninfected volunteers receiving SUSTIVA. In patients treated with SUSTIVA + zidovudine + lamivudine, increases from baseline in nonfasting total cholesterol and HDL of approximately 20% and 25%, respectively, were observed. In patients treated with SUSTIVA + indinavir, increases from baseline in nonfasting cholesterol and HDL of approximately 40% and 35%, respectively, were observed. Nonfasting total cholesterol levels ≥240 mg/dL and ≥300 mg/dL were reported in 34% and 9%, respectively, of patients treated with SUSTIVA + zidovudine + lamivudine; 54% and 20%, respectively, of patients treated with SUSTIVA + indinavir; and 28% and 4%, respectively, of patients treated with indinavir + zidovudine + lamivudine. The effects of SUSTIVA on triglycerides and LDL in this study were not well characterized since samples were taken from nonfasting patients. The clinical significance of these findings is unknown [see Warnings and Precautions (5.10)].
6.2 Clinical Trial Experience in Pediatric Patients
Clinical adverse experiences observed in ≥10% of 57 pediatric patients aged 3 to 16 years who received SUSTIVA capsules, nelfinavir, and one or more NRTIs in Study ACTG 382 [see Use In Specific Populations (8.4)] were rash (46%), diarrhea/loose stools (39%), fever (21%), cough (16%), dizziness/lightheaded/fainting (16%), ache/pain/discomfort (14%), nausea/vomiting (12%), and headache (11%). The incidence of nervous system symptoms was 18% (10/57). One patient experienced Grade 3 rash, two patients had Grade 4 rash, and five patients (9%) discontinued because of rash [see Warnings and Precautions (5.7) and Adverse Reactions (6.1, Table 5)].
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of SUSTIVA. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: allergic reactions, asthenia, redistribution/accumulation of body fat [see Warnings and Precautions (5.12)]
Central and Peripheral Nervous System: abnormal coordination, ataxia, cerebellar coordination and balance disturbances, convulsions, hypoesthesia, paresthesia, neuropathy, tremor, vertigo
Endocrine: gynecomastia
Gastrointestinal: constipation, malabsorption
Cardiovascular: flushing, palpitations
Liver and Biliary System: hepatic enzyme increase, hepatic failure, hepatitis. A few of the postmarketing reports of hepatic failure, including cases in patients with no pre-existing hepatic disease or other identifiable risk factors, were characterized by a fulminant course, progressing in some cases to transplantation or death.
Metabolic and Nutritional: hypercholesterolemia, hypertriglyceridemia
Musculoskeletal: arthralgia, myalgia, myopathy
Psychiatric: aggressive reactions, agitation, delusions, emotional lability, mania, neurosis, paranoia, psychosis, suicide
Respiratory: dyspnea
Skin and Appendages: erythema multiforme, photoallergic dermatitis, Stevens-Johnson syndrome
Special Senses: abnormal vision, tinnitus
-
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
Efavirenz has been shown in vivo to induce CYP3A and CYP2B6. Other compounds that are substrates of CYP3A or CYP2B6 may have decreased plasma concentrations when coadministered with SUSTIVA. In vitro studies have demonstrated that efavirenz inhibits CYP2C9, 2C19, and 3A4 isozymes in the range of observed efavirenz plasma concentrations. Coadministration of efavirenz with drugs primarily metabolized by these isozymes may result in altered plasma concentrations of the coadministered drug. Therefore, appropriate dose adjustments may be necessary for these drugs.
Drugs that induce CYP3A activity (eg, phenobarbital, rifampin, rifabutin) would be expected to increase the clearance of efavirenz resulting in lowered plasma concentrations. Drug interactions with SUSTIVA are summarized in Tables 2 and 7 [for pharmacokinetics data see Clinical Pharmacology (12.3, Tables 8 and 9)]. The tables include potentially significant interactions, but are not all inclusive.
Table 7: Established and Other Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interaction Concomitant Drug Class: Drug Name Effect Clinical Comment * The interaction between SUSTIVA and the drug was evaluated in a clinical study. All other drug interactions shown are predicted. This table is not all-inclusive. HIV antiviral agents Protease inhibitor:
Fosamprenavir
calcium
↓ amprenavirFosamprenavir (unboosted): Appropriate doses of the combinations with respect to safety and efficacy have not been established.
Fosamprenavir/ritonavir: An additional 100 mg/day (300 mg total) of ritonavir is recommended when SUSTIVA is administered with fosamprenavir/ritonavir once daily. No change in the ritonavir dose is required when SUSTIVA is administered with fosamprenavir plus ritonavir twice daily.Protease inhibitor:
Atazanavir sulfate
↓ atazanavir*Treatment-naive patients: When coadministered with SUSTIVA, the recommended dose of atazanavir is 400 mg with ritonavir 100 mg (together once daily with food) and SUSTIVA 600 mg (once daily on an empty stomach, preferably at bedtime).
Treatment-experienced patients: Coadministration of SUSTIVA and atazanavir is not recommended.Protease inhibitor:
Indinavir
↓ indinavir*The optimal dose of indinavir, when given in combination with SUSTIVA, is not known. Increasing the indinavir dose to 1000 mg every 8 hours does not compensate for the increased indinavir metabolism due to SUSTIVA. When indinavir at an increased dose (1000 mg every 8 hours) was given with SUSTIVA (600 mg once daily), the indinavir AUC and Cmin were decreased on average by 33-46% and 39-57%, respectively, compared to when indinavir (800 mg every 8 hours) was given alone. Protease inhibitor:
Lopinavir/ritonavir
↓ lopinavir*Lopinavir/ritonavir tablets should not be administered once daily in combination with SUSTIVA. In antiretroviral-naive patients, lopinavir/ritonavir tablets can be used twice daily in combination with SUSTIVA with no dose adjustment. A dose increase of lopinavir/ritonavir tablets to 600/150 mg (3 tablets) twice daily may be considered when used in combination with SUSTIVA in treatment-experienced patients where decreased susceptibility to lopinavir is clinically suspected (by treatment history or laboratory evidence). A dose increase of lopinavir/ritonavir oral solution to 533/133 mg (6.5 mL) twice daily taken with food is recommended when used in combination with SUSTIVA. Protease inhibitor:
Ritonavir
↑ ritonavir*
↑ efavirenz*When ritonavir 500 mg q12h was coadministered with SUSTIVA 600 mg once daily, the combination was associated with a higher frequency of adverse clinical experiences (eg, dizziness, nausea, paresthesia) and laboratory abnormalities (elevated liver enzymes). Monitoring of liver enzymes is recommended when SUSTIVA is used in combination with ritonavir. Protease inhibitor:
Saquinavir
↓ saquinavir*Should not be used as sole protease inhibitor in combination with SUSTIVA. NNRTI:
Other NNRTIs↑ or ↓ efavirenz
and/or NNRTICombining two NNRTIs has not been shown to be beneficial. SUSTIVA should not be coadministered with other NNRTIs. CCR5 co-receptor antagonist:
Maraviroc
↓ maraviroc*Refer to the full prescribing information for maraviroc for guidance on coadministration with efavirenz. Integrase strand transfer inhibitor:
Raltegravir
↓ raltegravir*SUSTIVA reduces plasma concentrations of raltegravir. The clinical significance of this interaction has not been directly assessed. Hepatitis C antiviral agents Protease inhibitor:
Boceprevir
↓ boceprevir*Plasma trough concentrations of boceprevir were decreased when boceprevir was coadministered with SUSTIVA, which may result in loss of therapeutic effect. The combination should be avoided. Protease inhibitor:
Telaprevir
↓ telaprevir*
↓ efavirenz*Concomitant administration of telaprevir and SUSTIVA resulted in reduced steady-state exposures to telaprevir and efavirenz. Other agents Anticoagulant:
Warfarin
↑ or ↓ warfarinPlasma concentrations and effects potentially increased or decreased by SUSTIVA. Anticonvulsants:
Carbamazepine
↓ carbamazepine*
↓ efavirenz*
There are insufficient data to make a dose recommendation for efavirenz. Alternative anticonvulsant treatment should be used.Phenytoin
Phenobarbital↓ anticonvulsant
↓ efavirenzPotential for reduction in anticonvulsant and/or efavirenz plasma levels; periodic monitoring of anticonvulsant plasma levels should be conducted. Antidepressants:
Bupropion
↓ bupropion*
The effect of efavirenz on bupropion exposure is thought to be due to the induction of bupropion metabolism. Increases in bupropion dosage should be guided by clinical response, but the maximum recommended dose of bupropion should not be exceeded.Sertraline ↓ sertraline* Increases in sertraline dosage should be guided by clinical response. Antifungals:
Voriconazole
↓ voriconazole*
↑ efavirenz*
SUSTIVA and voriconazole must not be coadministered at standard doses. Efavirenz significantly decreases voriconazole plasma concentrations, and coadministration may decrease the therapeutic effectiveness of voriconazole. Also, voriconazole significantly increases efavirenz plasma concentrations, which may increase the risk of SUSTIVA-associated side effects. When voriconazole is coadministered with SUSTIVA, voriconazole maintenance dose should be increased to 400 mg every 12 hours and SUSTIVA dose should be decreased to 300 mg once daily using the capsule formulation. SUSTIVA tablets should not be broken. [See Dosage and Administration (2.1) and Clinical Pharmacology (12.3, Tables 8 and 9).]
Itraconazole
↓ itraconazole*
↓ hydroxyitraconazole*
Since no dose recommendation for itraconazole can be made, alternative antifungal treatment should be considered.Ketoconazole ↓ ketoconazole Drug interaction studies with SUSTIVA and ketoconazole have not been conducted. SUSTIVA has the potential to decrease plasma concentrations of ketoconazole. Posaconazole ↓ posaconazole* Avoid concomitant use unless the benefit outweighs the risks. Anti-infective:
Clarithromycin
↓ clarithromycin*
↑ 14-OH metabolite*Plasma concentrations decreased by SUSTIVA; clinical significance unknown. In uninfected volunteers, 46% developed rash while receiving SUSTIVA and clarithromycin. No dose adjustment of SUSTIVA is recommended when given with clarithromycin. Alternatives to clarithromycin, such as azithromycin, should be considered (see Other Drugs, following table). Other macrolide antibiotics, such as erythromycin, have not been studied in combination with SUSTIVA. Antimycobacterials:
Rifabutin
↓ rifabutin*Increase daily dose of rifabutin by 50%. Consider doubling the rifabutin dose in regimens where rifabutin is given 2 or 3 times a week. Rifampin ↓ efavirenz* If SUSTIVA is coadministered with rifampin to patients weighing 50 kg or more, an increase in the dose of SUSTIVA to 800 mg once daily is recommended. Calcium channel blockers:
Diltiazem
↓ diltiazem*
↓ desacetyl diltiazem*
↓ N-monodesmethyl diltiazem*Diltiazem dose adjustments should be guided by clinical response (refer to the full prescribing information for diltiazem). No dose adjustment of efavirenz is necessary when administered with diltiazem. Others (eg, felodipine, nicardipine, nifedipine, verapamil)
↓ calcium channel blockerNo data are available on the potential interactions of efavirenz with other calcium channel blockers that are substrates of CYP3A. The potential exists for reduction in plasma concentrations of the calcium channel blocker. Dose adjustments should be guided by clinical response (refer to the full prescribing information for the calcium channel blocker). HMG-CoA reductase inhibitors:
Atorvastatin
Pravastatin
Simvastatin
↓ atorvastatin*
↓ pravastatin*
↓ simvastatin*Plasma concentrations of atorvastatin, pravastatin, and simvastatin decreased. Consult the full prescribing information for the HMG-CoA reductase inhibitor for guidance on individualizing the dose. Hormonal contraceptives:
Oral
Ethinyl estradiol/
Norgestimate
↓ active metabolites
of norgestimate*
A reliable method of barrier contraception must be used in addition to hormonal contraceptives. Efavirenz had no effect on ethinyl estradiol concentrations, but progestin levels (norelgestromin and levonorgestrel) were markedly decreased. No effect of ethinyl estradiol/norgestimate on efavirenz plasma concentrations was observed.Implant
Etonogestrel
↓ etonogestrel
A reliable method of barrier contraception must be used in addition to hormonal contraceptives. The interaction between etonogestrel and efavirenz has not been studied. Decreased exposure of etonogestrel may be expected. There have been postmarketing reports of contraceptive failure with etonogestrel in efavirenz-exposed patients.Immunosuppressants:
Cyclosporine, tacrolimus, sirolimus, and others metabolized by CYP3A
↓ immunosuppressantDecreased exposure of the immunosuppressant may be expected due to CYP3A induction. These immunosuppressants are not anticipated to affect exposure of efavirenz. Dose adjustments of the immunosuppressant may be required. Close monitoring of immunosuppressant concentrations for at least 2 weeks (until stable concentrations are reached) is recommended when starting or stopping treatment with efavirenz. Narcotic analgesic:
Methadone
↓ methadone*Coadministration in HIV-infected individuals with a history of injection drug use resulted in decreased plasma levels of methadone and signs of opiate withdrawal. Methadone dose was increased by a mean of 22% to alleviate withdrawal symptoms. Patients should be monitored for signs of withdrawal and their methadone dose increased as required to alleviate withdrawal symptoms. Other Drugs
Based on the results of drug interaction studies [see Clinical Pharmacology (12.3, Tables 8 and 9)], no dosage adjustment is recommended when SUSTIVA (efavirenz) is given with the following: aluminum/magnesium hydroxide antacids, azithromycin, cetirizine, famotidine, fluconazole, lamivudine, lorazepam, nelfinavir, paroxetine, tenofovir disoproxil fumarate, and zidovudine.
Specific drug interaction studies have not been performed with SUSTIVA and NRTIs other than lamivudine and zidovudine. Clinically significant interactions would not be expected since the NRTIs are metabolized via a different route than efavirenz and would be unlikely to compete for the same metabolic enzymes and elimination pathways.
7.2 Cannabinoid Test Interaction
Efavirenz does not bind to cannabinoid receptors. False-positive urine cannabinoid test results have been observed in non-HIV-infected volunteers receiving SUSTIVA when the Microgenics CEDIA DAU Multi-Level THC assay was used for screening. Negative results were obtained when more specific confirmatory testing was performed with gas chromatography/mass spectrometry.
Of the three assays analyzed (Microgenics CEDIA DAU Multi-Level THC assay, Cannabinoid Enzyme Immunoassay [Diagnostic Reagents, Inc], and AxSYM Cannabinoid Assay), only the Microgenics CEDIA DAU Multi-Level THC assay showed false-positive results. The other two assays provided true-negative results. The effects of SUSTIVA on cannabinoid screening tests other than these three are unknown. The manufacturers of cannabinoid assays should be contacted for additional information regarding the use of their assays with patients receiving efavirenz.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D: See Warnings and Precautions (5.6).
Antiretroviral Pregnancy Registry: To monitor fetal outcomes of pregnant women exposed to SUSTIVA, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
As of July 2010, the Antiretroviral Pregnancy Registry has received prospective reports of 792 pregnancies exposed to efavirenz-containing regimens, nearly all of which were first-trimester exposures (718 pregnancies). Birth defects occurred in 17 of 604 live births (first-trimester exposure) and 2 of 69 live births (second/third-trimester exposure). One of these prospectively reported defects with first-trimester exposure was a neural tube defect. A single case of anophthalmia with first-trimester exposure to efavirenz has also been prospectively reported; however, this case included severe oblique facial clefts and amniotic banding, a known association with anophthalmia. There have been six retrospective reports of findings consistent with neural tube defects, including meningomyelocele. All mothers were exposed to efavirenz-containing regimens in the first trimester. Although a causal relationship of these events to the use of SUSTIVA has not been established, similar defects have been observed in preclinical studies of efavirenz.
Animal Data
Effects of efavirenz on embryo-fetal development have been studied in three nonclinical species (cynomolgus monkeys, rats, and rabbits). In monkeys, efavirenz 60 mg/kg/day was administered to pregnant females throughout pregnancy (gestation days 20 through 150). The maternal systemic drug exposures (AUC) were 1.3 times the exposure in humans at the recommended clinical dose (600 mg/day), with fetal umbilical venous drug concentrations approximately 0.7 times the maternal values. Three fetuses of 20 fetuses/infants had one or more malformations; there were no malformed fetuses or infants from placebo-treated mothers. The malformations that occurred in these three monkey fetuses included anencephaly and unilateral anophthalmia in one fetus, microophthalmia in a second, and cleft palate in the third. There was no NOAEL (no observable adverse effect level) established for this study because only one dosage was evaluated. In rats, efavirenz was administered either during organogenesis (gestation days 7 to 18) or from gestation day 7 through lactation day 21 at 50, 100, or 200 mg/kg/day. Administration of 200 mg/kg/day in rats was associated with increase in the incidence of early resorptions; and doses 100 mg/kg/day and greater were associated with early neonatal mortality. The AUC at the NOAEL (50 mg/kg/day) in this rat study was 0.1 times that in humans at the recommended clinical dose. Drug concentrations in the milk on lactation day 10 were approximately 8 times higher than those in maternal plasma. In pregnant rabbits, efavirenz was neither embryo lethal nor teratogenic when administered at doses of 25, 50, and 75 mg/kg/day over the period of organogenesis (gestation days 6 through 18). The AUC at the NOAEL (75 mg/kg/day) in rabbits was 0.4 times that in humans at the recommended clinical dose.
8.3 Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV. Although it is not known if efavirenz is secreted in human milk, efavirenz is secreted into the milk of lactating rats. Because of the potential for HIV transmission and the potential for serious adverse effects in nursing infants, mothers should be instructed not to breast-feed if they are receiving SUSTIVA.
8.4 Pediatric Use
ACTG 382 is an ongoing, open-label study in 57 NRTI-experienced pediatric patients to characterize the safety, pharmacokinetics, and antiviral activity of SUSTIVA in combination with nelfinavir (20-30 mg/kg three times daily) and NRTIs. Mean age was 8 years (range 3-16). SUSTIVA has not been studied in pediatric patients below 3 years of age or who weigh less than 13 kg. At 48 weeks, the type and frequency of adverse experiences was generally similar to that of adult patients with the exception of a higher incidence of rash, which was reported in 46% (26/57) of pediatric patients compared to 26% of adults, and a higher frequency of Grade 3 or 4 rash reported in 5% (3/57) of pediatric patients compared to 0.9% of adults [see Warnings and Precautions (5.7) and Adverse Reactions (6.1, Table 5; 6.2)].
The starting dose of SUSTIVA was 600 mg once daily adjusted to body size, based on weight, targeting AUC levels in the range of 190-380 µM•h [see Dosage and Administration (2.2)]. The pharmacokinetics of efavirenz in pediatric patients were similar to the pharmacokinetics in adults who received 600-mg daily doses of SUSTIVA. In 48 pediatric patients receiving the equivalent of a 600-mg dose of SUSTIVA, steady-state Cmax was 14.2 ± 5.8 µM (mean ± SD), steady-state Cmin was 5.6 ± 4.1 µM, and AUC was 218 ± 104 µM•h.
8.5 Geriatric Use
Clinical studies of SUSTIVA did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other therapy.
8.6 Hepatic Impairment
SUSTIVA is not recommended for patients with moderate or severe hepatic impairment because there are insufficient data to determine whether dose adjustment is necessary. Patients with mild hepatic impairment may be treated with efavirenz without any adjustment in dose. Because of the extensive cytochrome P450-mediated metabolism of efavirenz and limited clinical experience in patients with hepatic impairment, caution should be exercised in administering SUSTIVA to these patients [see Warnings and Precautions (5.8) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Some patients accidentally taking 600 mg twice daily have reported increased nervous system symptoms. One patient experienced involuntary muscle contractions.
Treatment of overdose with SUSTIVA should consist of general supportive measures, including monitoring of vital signs and observation of the patient’s clinical status. Administration of activated charcoal may be used to aid removal of unabsorbed drug. There is no specific antidote for overdose with SUSTIVA. Since efavirenz is highly protein bound, dialysis is unlikely to significantly remove the drug from blood.
-
11 DESCRIPTION
SUSTIVA® (efavirenz) is an HIV-1 specific, non-nucleoside, reverse transcriptase inhibitor (NNRTI). Efavirenz is chemically described as (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one. Its empirical formula is C14H9ClF3NO2 and its structural formula is:
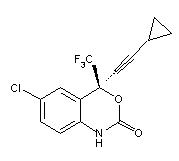
Efavirenz is a white to slightly pink crystalline powder with a molecular mass of 315.68. It is practically insoluble in water (<10 microgram/mL).
Capsules: SUSTIVA is available as capsules for oral administration containing either 50 mg or 200 mg of efavirenz and the following inactive ingredients: lactose monohydrate, magnesium stearate, sodium lauryl sulfate, and sodium starch glycolate. The capsule shell contains the following inactive ingredients and dyes: gelatin, sodium lauryl sulfate, titanium dioxide, and/or yellow iron oxide. The capsule shells may also contain silicon dioxide. The capsules are printed with ink containing carmine 40 blue, FD&C Blue No. 2, and titanium dioxide.
Tablets: SUSTIVA is available as film-coated tablets for oral administration containing 600 mg of efavirenz and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The film coating contains Opadry Yellow and Opadry Clear. The tablets are polished with carnauba wax and printed with purple ink, Opacode WB.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Absorption
Peak efavirenz plasma concentrations of 1.6-9.1 μM were attained by 5 hours following single oral doses of 100 mg to 1600 mg administered to uninfected volunteers. Dose-related increases in Cmax and AUC were seen for doses up to 1600 mg; the increases were less than proportional suggesting diminished absorption at higher doses.
In HIV-1-infected patients at steady state, mean Cmax, mean Cmin, and mean AUC were dose proportional following 200-mg, 400-mg, and 600-mg daily doses. Time-to-peak plasma concentrations were approximately 3-5 hours and steady-state plasma concentrations were reached in 6-10 days. In 35 patients receiving SUSTIVA 600 mg once daily, steady-state Cmax was 12.9 ± 3.7 μM (mean ± SD), steady-state Cmin was 5.6 ± 3.2 μM, and AUC was 184 ± 73 μM•h.
Effect of Food on Oral Absorption:
Capsules: Administration of a single 600-mg dose of efavirenz capsules with a high-fat/high-caloric meal (894 kcal, 54 g fat, 54% calories from fat) or a reduced-fat/normal-caloric meal (440 kcal, 2 g fat, 4% calories from fat) was associated with a mean increase of 22% and 17% in efavirenz AUC∞ and a mean increase of 39% and 51% in efavirenz Cmax, respectively, relative to the exposures achieved when given under fasted conditions. See Dosage and Administration (2) and Patient Counseling Information (17.3).
Tablets: Administration of a single 600-mg efavirenz tablet with a high-fat/high-caloric meal (approximately 1000 kcal, 500-600 kcal from fat) was associated with a 28% increase in mean AUC∞ of efavirenz and a 79% increase in mean Cmax of efavirenz relative to the exposures achieved under fasted conditions. See Dosage and Administration (2) and Patient Counseling Information (17.3).
Distribution
Efavirenz is highly bound (approximately 99.5-99.75%) to human plasma proteins, predominantly albumin. In HIV-1 infected patients (n=9) who received SUSTIVA 200 to 600 mg once daily for at least one month, cerebrospinal fluid concentrations ranged from 0.26 to 1.19% (mean 0.69%) of the corresponding plasma concentration. This proportion is approximately 3-fold higher than the non-protein-bound (free) fraction of efavirenz in plasma.
Metabolism
Studies in humans and in vitro studies using human liver microsomes have demonstrated that efavirenz is principally metabolized by the cytochrome P450 system to hydroxylated metabolites with subsequent glucuronidation of these hydroxylated metabolites. These metabolites are essentially inactive against HIV-1. The in vitro studies suggest that CYP3A and CYP2B6 are the major isozymes responsible for efavirenz metabolism.
Efavirenz has been shown to induce CYP enzymes, resulting in the induction of its own metabolism. Multiple doses of 200-400 mg per day for 10 days resulted in a lower than predicted extent of accumulation (22-42% lower) and a shorter terminal half-life of 40-55 hours (single dose half-life 52-76 hours).
Elimination
Efavirenz has a terminal half-life of 52-76 hours after single doses and 40-55 hours after multiple doses. A one-month mass balance/excretion study was conducted using 400 mg per day with a 14C-labeled dose administered on Day 8. Approximately 14-34% of the radiolabel was recovered in the urine and 16-61% was recovered in the feces. Nearly all of the urinary excretion of the radiolabeled drug was in the form of metabolites. Efavirenz accounted for the majority of the total radioactivity measured in feces.
Special Populations
Gender and race: The pharmacokinetics of efavirenz in patients appear to be similar between men and women and among the racial groups studied.
Renal impairment: The pharmacokinetics of efavirenz have not been studied in patients with renal insufficiency; however, less than 1% of efavirenz is excreted unchanged in the urine, so the impact of renal impairment on efavirenz elimination should be minimal.
Hepatic impairment: A multiple-dose study showed no significant effect on efavirenz pharmacokinetics in patients with mild hepatic impairment (Child-Pugh Class A) compared with controls. There were insufficient data to determine whether moderate or severe hepatic impairment (Child-Pugh Class B or C) affects efavirenz pharmacokinetics.
Drug Interaction Studies
Efavirenz has been shown in vivo to cause hepatic enzyme induction, thus increasing the biotransformation of some drugs metabolized by CYP3A and CYP2B6. In vitro studies have shown that efavirenz inhibited CYP isozymes 2C9, 2C19, and 3A4 with Ki values (8.5-17 μM) in the range of observed efavirenz plasma concentrations. In in vitro studies, efavirenz did not inhibit CYP2E1 and inhibited CYP2D6 and CYP1A2 (Ki values 82-160 μM) only at concentrations well above those achieved clinically. The inhibitory effect on CYP3A is expected to be similar between 200-mg, 400-mg, and 600-mg doses of efavirenz. Coadministration of efavirenz with drugs primarily metabolized by 2C9, 2C19, and 3A isozymes may result in altered plasma concentrations of the coadministered drug. Drugs which induce CYP3A activity would be expected to increase the clearance of efavirenz resulting in lowered plasma concentrations.
Drug interaction studies were performed with efavirenz and other drugs likely to be coadministered or drugs commonly used as probes for pharmacokinetic interaction. The effects of coadministration of efavirenz on the Cmax, AUC, and Cmin are summarized in Table 8 (effect of efavirenz on other drugs) and Table 9 (effect of other drugs on efavirenz). For information regarding clinical recommendations see Contraindications (4.2) and Drug Interactions (7.1).
Table 8: Effect of Efavirenz on Coadministered Drug Plasma Cmax, AUC, and Cmin Coadministered Drug Dose Efavirenz Dose Number of Subjects Coadministered Drug
(mean % change)Cmax
(90% CI)AUC
(90% CI)Cmin
(90% CI)↑ Indicates increase ↓ Indicates decrease ↔ Indicates no change or a mean increase or decrease of <10%. a Compared with atazanavir 400 mg qd alone. b Comparator dose of indinavir was 800 mg q8h x 10 days. c Parallel-group design; n for efavirenz + lopinavir/ritonavir, n for lopinavir/ritonavir alone. d Values are for lopinavir; the pharmacokinetics of ritonavir in this study were unaffected by concurrent efavirenz. e 95% CI. f Soft Gelatin Capsule. g Tenofovir disoproxil fumarate. h 90% CI not available. i Relative to steady-state administration of voriconazole (400 mg for 1 day, then 200 mg po q12h for 2 days). j Not available because of insufficient data. NA = not available. Atazanavir 400 mg qd with a light meal d 1‑20 600 mg qd with a light meal d 7‑20 27 ↓ 59%
(49-67%)↓ 74%
(68-78%)↓ 93%
(90-95%)400 mg qd d 1‑6, then 300 mg qd d 7‑20 with ritonavir 100 mg qd and a light meal 600 mg qd 2 h after atazanavir and ritonavir d 7‑20 13 ↑ 14%a
(↓ 17-↑ 58%)↑ 39%a
(2-88%)↑ 48%a
(24-76%)300 mg qd/ritonavir 100 mg qd d 1‑10 (pm), then 400 mg qd/ritonavir 100 mg qd d 11‑24 (pm) (simultaneous with efavirenz) 600 mg qd with a light snack d 11‑24 (pm) 14 ↑ 17%
(8-27%)↔ ↓ 42%
(31-51%)Indinavir 1000 mg q8h x 10 days 600 mg qd x 10 days 20 After morning dose ↔b ↓ 33%b
(26-39%)↓ 39%b
(24-51%)After afternoon dose ↔b ↓ 37%b
(26-46%)↓ 52%b
(47-57%)After evening dose ↓ 29%b
(11-43%)↓ 46%b
(37-54%)↓ 57%b
(50-63%)Lopinavir/
ritonavir400/100 mg capsule
q12h x 9 days600 mg qd x 9 days 11,7c ↔d ↓ 19%d
(↓ 36-↑ 3%)↓ 39%d
(3-62%)600/150 mg tablet
q12h x 10 days with
efavirenz compared to
400/100 mg q12h alone600 mg qd x 9 days 23 ↑ 36%d
(28-44%)↑ 36%d
(28-44%)↑ 32%d
(21-44%)Nelfinavir 750 mg q8h x 7 days 600 mg qd x 7 days 10 ↑ 21%
(10-33%)↑ 20%
(8-34%)↔ Metabolite
AG-1402↓ 40%
(30-48%)↓ 37%
(25-48%)↓ 43%
(21-59%)Ritonavir 500 mg q12h x 8 days 600 mg qd x 10 days 11 After AM dose ↑ 24%
(12-38%)↑ 18%
(6-33%)↑ 42%
(9-86%)eAfter PM dose ↔ ↔ ↑ 24%
(3-50%)eSaquinavir
SGCf1200 mg q8h x 10 days 600 mg qd x 10 days 12 ↓ 50%
(28-66%)↓ 62%
(45-74%)↓ 56%
(16-77%)eLamivudine 150 mg q12h x 14 days 600 mg qd x 14 days 9 ↔ ↔ ↑ 265%
(37-873%)Tenofovirg 300 mg qd 600 mg qd x 14 days 29 ↔ ↔ ↔ Zidovudine 300 mg q12h x 14 days 600 mg qd x 14 days 9 ↔ ↔ ↑ 225%
(43-640%)Maraviroc 100 mg bid 600 mg qd 12 ↓ 51%
(37-62%)↓ 45%
(38-51%)↓ 45%
(28-57%)Raltegravir 400 mg single dose 600 mg qd 9 ↓ 36%
(2-59%)↓ 36%
(20-48%)↓ 21%
(↓ 51-↑ 28%)Boceprevir 800 mg tid x 6 days 600 mg qd x 16 days NA ↓ 8%
(↓ 22-↑ 8%)↓ 19%
(11-25%)↓ 44%
(26-58%)Telaprevir 750 mg q8h x 10 days 600 mg qd x 20 days 21 ↓ 9%
(↓ 18-↑ 2%)↓ 26%
(16-35%)↓ 47%
(35-56%)Azithromycin 600 mg single dose 400 mg qd x 7 days 14 ↑ 22%
(4-42%)↔ NA Clarithromycin 500 mg q12h x 7 days 400 mg qd x 7 days 11 ↓ 26%
(15-35%)↓ 39%
(30-46%)↓ 53%
(42-63%)14-OH metabolite ↑ 49%
(32-69%)↑ 34%
(18-53%)↑ 26%
(9-45%)Fluconazole 200 mg x 7 days 400 mg qd x 7 days 10 ↔ ↔ ↔ Itraconazole 200 mg q12h x 28 days 600 mg qd x 14 days 18 ↓ 37%
(20-51%)↓ 39%
(21-53%)↓ 44%
(27-58%)Hydroxy-itraconazole ↓ 35%
(12-52%)↓ 37%
(14-55%)↓ 43%
(18-60%)Posaconazole 400 mg (oral suspension) bid x 10 and 20 days 400 mg qd x 10 and 20 days 11 ↓ 45%
(34-53%)↓ 50%
(40-57%)NA Rifabutin 300 mg qd x 14 days 600 mg qd x 14 days 9 ↓ 32%
(15-46%)↓ 38%
(28-47%)↓ 45%
(31-56%)Voriconazole 400 mg po q12h x 1 day, then 200 mg po q12h x 8 days 400 mg qd x 9 days NA ↓ 61%h ↓ 77%h NA 300 mg po q12h days 2‑7 300 mg qd x 7 days NA ↓ 36%i
(21-49%)↓ 55%i
(45-62%)NA 400 mg po q12h days 2‑7 300 mg qd x 7 days NA ↑ 23%i
(↓ 1-↑ 53%)↓ 7%i
(↓ 23-↑ 13%)NA Atorvastatin 10 mg qd x 4 days 600 mg qd x 15 days 14 ↓ 14%
(1-26%)↓ 43%
(34-50%)↓ 69%
(49-81%)Total active
(including
metabolites)↓ 15%
(2-26%)↓ 32%
(21-41%)↓ 48%
(23-64%)Pravastatin 40 mg qd x 4 days 600 mg qd x 15 days 13 ↓ 32%
(↓ 59-↑ 12%)↓ 44%
(26-57%)↓ 19%
(0-35%)Simvastatin 40 mg qd x 4 days 600 mg qd x 15 days 14 ↓ 72%
(63-79%)↓ 68%
(62-73%)↓ 45%
(20-62%)Total active
(including
metabolites)↓ 68%
(55-78%)↓ 60%
(52-68%)NAj Carbamazepine 200 mg qd x 3 days, 200 mg bid x 3 days, then 400 mg qd x 29 days 600 mg qd x 14 days 12 ↓ 20%
(15-24%)↓ 27%
(20-33%)↓ 35%
(24-44%)Epoxide metabolite ↔ ↔ ↓ 13%
(↓ 30-↑ 7%)Cetirizine 10 mg single dose 600 mg qd x 10 days 11 ↓ 24%
(18-30%)↔ NA Diltiazem 240 mg x 21 days 600 mg qd x 14 days 13 ↓ 60%
(50-68%)↓ 69%
(55-79%)↓ 63%
(44-75%)Desacetyl
diltiazem↓ 64%
(57-69%)↓ 75%
(59-84%)↓ 62%
(44-75%)N-
monodesmethyl
diltiazem↓ 28%
(7-44%)↓ 37%
(17-52%)↓ 37%
(17-52%)Ethinyl estradiol/
Norgestimate0.035 mg/
0.25 mg x 14 days600 mg qd x 14 days Ethinyl
estradiol21 ↔ ↔ ↔ Norelgestromin 21 ↓ 46%
(39-52%)↓ 64%
(62-67%)↓ 82%
(79-85%)Levonorgestrel 6 ↓ 80%
(77-83%)↓ 83%
(79-87%)↓ 86%
(80-90%)Lorazepam 2 mg single dose 600 mg qd x 10 days 12 ↑ 16%
(2-32%)↔ NA Methadone Stable
maintenance 35-
100 mg daily600 mg qd x 14-21 days 11 ↓ 45%
(25-59%)↓ 52%
(33-66%)NA Bupropion 150 mg single dose
(sustained-release)600 mg qd x 14 days 13 ↓ 34%
(21-47%)↓ 55%
(48-62%)NA Hydroxy-
bupropion↑ 50%
(20-80%)↔ NA Paroxetine 20 mg qd x 14 days 600 mg qd x 14 days 16 ↔ ↔ ↔ Sertraline 50 mg qd x 14 days 600 mg qd x 14 days 13 ↓ 29%
(15-40%)↓ 39%
(27-50%)↓ 46%
(31-58%)Table 9: Effect of Coadministered Drug on Efavirenz Plasma Cmax, AUC, and Cmin Efavirenz
(mean % change)Coadministered Drug Dose Efavirenz Dose Number of Subjects Cmax
(90% CI)AUC
(90% CI)Cmin
(90% CI)↑ Indicates increase ↓ Indicates decrease ↔ Indicates no change or a mean increase or decrease of <10%. a Parallel-group design; n for efavirenz + lopinavir/ritonavir, n for efavirenz alone. b 95% CI. c Soft Gelatin Capsule. d Tenofovir disoproxil fumarate. e 90% CI not available. f Relative to steady-state administration of efavirenz (600 mg once daily for 9 days). NA = not available. Indinavir 800 mg q8h x 14 days 200 mg qd x 14 days 11 ↔ ↔ ↔ Lopinavir/
ritonavir400/100 mg q12h x 9 days 600 mg qd x 9 days 11,12a ↔ ↓ 16%
(↓ 38-↑ 15%)↓ 16%
(↓ 42-↑ 20%)Nelfinavir 750 mg q8h x 7 days 600 mg qd x 7 days 10 ↓ 12%
(↓ 32-↑ 13%)b↓ 12%
(↓ 35-↑ 18%)b↓ 21%
(↓ 53-↑ 33%)Ritonavir 500 mg q12h x 8 days 600 mg qd x 10 days 9 ↑ 14%
(4-26%)↑ 21%
(10-34%)↑ 25%
(7-46%)bSaquinavir
SGCc1200 mg q8h x 10 days 600 mg qd x 10 days 13 ↓ 13%
(5-20%)↓ 12%
(4-19%)↓ 14%
(2-24%)bTenofovird 300 mg qd 600 mg qd x 14 days 30 ↔ ↔ ↔ Boceprevir 800 mg tid x 6 days 600 mg qd x 16 days NA ↑ 11%
(2-20%)↑ 20%
(15-26%)NA Telaprevir 750 mg q8h x 10 days 600 mg qd x 20 days 21 ↓ 16%
(7-24%)↓ 7%
(2-13%)↓ 2%
(↓ 6-↑ 2%)Telaprevir, coadministered with tenofovir disoproxil fumarate (TDF) 1125 mg q8h x 7 days 600 mg efavirenz/300 mg TDF qd x 7 days 15 ↓ 24%
(15-32%)↓ 18%
(10-26%)↓ 10%
(↓ 19-↑ 1%)1500 mg q12h x 7 days 600 mg efavirenz/300 mg TDF qd x 7 days 16 ↓ 20%
(14-26%)↓ 15%
(9-21%)↓ 11%
(4-18%)Azithromycin 600 mg single dose 400 mg qd x 7 days 14 ↔ ↔ ↔ Clarithromycin 500 mg q12h x 7 days 400 mg qd x 7 days 12 ↑ 11%
(3-19%)↔ ↔ Fluconazole 200 mg x
7 days400 mg qd x 7 days 10 ↔ ↑ 16%
(6-26%)↑ 22%
(5-41%)Itraconazole 200 mg q12h x 14 days 600 mg qd x 28 days 16 ↔ ↔ ↔ Rifabutin 300 mg qd x 14 days 600 mg qd x 14 days 11 ↔ ↔ ↓ 12%
(↓ 24-↑ 1%)Rifampin 600 mg x
7 days600 mg qd x 7 days 12 ↓ 20%
(11-28%)↓ 26%
(15-36%)↓ 32%
(15-46%)Voriconazole 400 mg po q12h
x 1 day, then 200 mg po q12h
x 8 days400 mg qd x 9 days NA ↑ 38%e ↑ 44%e NA 300 mg po q12h days 2-7 300 mg qd x 7 days NA ↓ 14%f
(7-21%)↔f NA 400 mg po q12h days 2-7 300 mg qd x 7 days NA ↔f ↑ 17%f
(6-29%)NA Atorvastatin 10 mg qd x
4 days600 mg qd x 15 days 14 ↔ ↔ ↔ Pravastatin 40 mg qd x
4 days600 mg qd x 15 days 11 ↔ ↔ ↔ Simvastatin 40 mg qd x
4 days600 mg qd x 15 days 14 ↓ 12%
(↓ 28-↑ 8%)↔ ↓ 12%
(↓ 25-↑ 3%)Aluminum hydroxide 400 mg, magnesium hydroxide 400 mg, plus simethicone 40 mg 30 mL single dose 400 mg single dose 17 ↔ ↔ NA Carbamazepine 200 mg qd x 3 days, 200 mg
bid x 3 days, then 400 mg qd x 15 days600 mg qd x 35 days 14 ↓ 21%
(15-26%)↓ 36%
(32-40%)↓ 47%
(41-53%)Cetirizine 10 mg single dose 600 mg qd x 10 days 11 ↔ ↔ ↔ Diltiazem 240 mg x
14 days600 mg qd x 28 days 12 ↑ 16%
(6-26%)↑ 11%
(5-18%)↑ 13%
(1-26%)Famotidine 40 mg single dose 400 mg single dose 17 ↔ ↔ NA Paroxetine 20 mg qd x 14 days 600 mg qd x 14 days 12 ↔ ↔ ↔ Sertraline 50 mg qd x 14 days 600 mg qd x 14 days 13 ↑ 11%
(6-16%)↔ ↔ 12.4 Microbiology
Mechanism of Action
Efavirenz (EFV) is an NNRTI of HIV-1. EFV activity is mediated predominantly by noncompetitive inhibition of HIV-1 reverse transcriptase (RT). HIV-2 RT and human cellular DNA polymerases α, β, γ, and δ are not inhibited by EFV.
Antiviral Activity in Cell Culture
The concentration of EFV inhibiting replication of wild-type laboratory adapted strains and clinical isolates in cell culture by 90-95% (EC90-95) ranged from 1.7 to 25 nM in lymphoblastoid cell lines, peripheral blood mononuclear cells (PBMCs), and macrophage/monocyte cultures. EFV demonstrated antiviral activity against clade B and most non-clade B isolates (subtypes A, AE, AG, C, D, F, G, J, N), but had reduced antiviral activity against group O viruses. EFV demonstrated additive antiviral activity without cytotoxicity against HIV-1 in cell culture when combined with the NNRTIs delavirdine (DLV) and nevirapine (NVP), NRTIs (abacavir, didanosine, emtricitabine, lamivudine [LAM], stavudine, tenofovir, zalcitabine, zidovudine [ZDV]), PIs (amprenavir, indinavir [IDV], lopinavir, nelfinavir, ritonavir, saquinavir), and the fusion inhibitor enfuvirtide. EFV demonstrated additive to antagonistic antiviral activity in cell culture with atazanavir. EFV was not antagonistic with adefovir, used for the treatment of hepatitis B virus infection, or ribavirin, used in combination with interferon for the treatment of hepatitis C virus infection.
Resistance
In cell culture
In cell culture, HIV-1 isolates with reduced susceptibility to EFV (>380-fold increase in EC90 value) emerged rapidly in the presence of drug. Genotypic characterization of these viruses identified single amino acid substitutions L100I or V179D, double substitutions L100I/V108I, and triple substitutions L100I/V179D/Y181C in RT.
Clinical studies
Clinical isolates with reduced susceptibility in cell culture to EFV have been obtained. One or more RT substitutions at amino acid positions 98, 100, 101, 103, 106, 108, 188, 190, 225, and 227 were observed in patients failing treatment with EFV in combination with IDV, or with ZDV plus LAM. The mutation K103N was the most frequently observed. Long-term resistance surveillance (average 52 weeks, range 4-106 weeks) analyzed 28 matching baseline and virologic failure isolates. Sixty-one percent (17/28) of these failure isolates had decreased EFV susceptibility in cell culture with a median 88-fold change in EFV susceptibility (EC50 value) from reference. The most frequent NNRTI substitution to develop in these patient isolates was K103N (54%). Other NNRTI substitutions that developed included L100I (7%), K101E/Q/R (14%), V108I (11%), G190S/T/A (7%), P225H (18%), and M230I/L (11%).
Cross-Resistance
Cross-resistance among NNRTIs has been observed. Clinical isolates previously characterized as EFV-resistant were also phenotypically resistant in cell culture to DLV and NVP compared to baseline. DLV- and/or NVP-resistant clinical viral isolates with NNRTI resistance-associated substitutions (A98G, L100I, K101E/P, K103N/S, V106A, Y181X, Y188X, G190X, P225H, F227L, or M230L) showed reduced susceptibility to EFV in cell culture. Greater than 90% of NRTI-resistant clinical isolates tested in cell culture retained susceptibility to EFV.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term carcinogenicity studies in mice and rats were carried out with efavirenz. Mice were dosed with 0, 25, 75, 150, or 300 mg/kg/day for 2 years. Incidences of hepatocellular adenomas and carcinomas and pulmonary alveolar/bronchiolar adenomas were increased above background in females. No increases in tumor incidence above background were seen in males. There was no NOAEL in females established for this study because tumor findings occurred at all doses. AUC at the NOAEL (150 mg/kg) in the males was approximately 0.9 times that in humans at the recommended clinical dose. In the rat study, no increases in tumor incidence were observed at doses up to 100 mg/kg/day, for which AUCs were 0.1 (males) or 0.2 (females) times those in humans at the recommended clinical dose.
Mutagenesis
Efavirenz tested negative in a battery of in vitro and in vivo genotoxicity assays. These included bacterial mutation assays in S. typhimurium and E. coli, mammalian mutation assays in Chinese hamster ovary cells, chromosome aberration assays in human peripheral blood lymphocytes or Chinese hamster ovary cells, and an in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
Efavirenz did not impair mating or fertility of male or female rats, and did not affect sperm of treated male rats. The reproductive performance of offspring born to female rats given efavirenz was not affected. The AUCs at the NOAEL values in male (200 mg/kg) and female (100 mg/kg) rats were approximately ≤0.15 times that in humans at the recommended clinical dose.
13.2 Animal Toxicology
Nonsustained convulsions were observed in 6 of 20 monkeys receiving efavirenz at doses yielding plasma AUC values 4- to 13-fold greater than those in humans given the recommended dose [see Warnings and Precautions (5.9)].
-
14 CLINICAL STUDIES
Study 006, a randomized, open-label trial, compared SUSTIVA (600 mg once daily) + zidovudine (ZDV, 300 mg q12h) + lamivudine (LAM, 150 mg q12h) or SUSTIVA (600 mg once daily) + indinavir (IDV, 1000 mg q8h) with indinavir (800 mg q8h) + zidovudine (300 mg q12h) + lamivudine (150 mg q12h). Twelve hundred sixty-six patients (mean age 36.5 years [range 18-81], 60% Caucasian, 83% male) were enrolled. All patients were efavirenz-, lamivudine-, NNRTI-, and PI-naive at study entry. The median baseline CD4+ cell count was 320 cells/mm3 and the median baseline HIV-1 RNA level was 4.8 log10 copies/mL. Treatment outcomes with standard assay (assay limit 400 copies/mL) through 48 and 168 weeks are shown in Table 10. Plasma HIV RNA levels were quantified with standard (assay limit 400 copies/mL) and ultrasensitive (assay limit 50 copies/mL) versions of the AMPLICOR HIV-1 MONITOR assay. During the study, version 1.5 of the assay was introduced in Europe to enhance detection of non-clade B virus.
Table 10: Outcomes of Randomized Treatment Through 48 and 168 Weeks, Study 006 SUSTIVA + ZDV
+ LAM
(n=422)SUSTIVA + IDV
(n=429)IDV + ZDV + LAM
(n=415)Outcome Week 48 Week 168 Week 48 Week 168 Week 48 Week 168 a Patients achieved and maintained confirmed HIV-1 RNA <400 copies/mL through Week 48 or Week 168. b Includes patients who rebounded, patients who were on study at Week 48 and failed to achieve confirmed HIV-1 RNA <400 copies/mL at time of discontinuation, and patients who discontinued due to lack of efficacy. c Includes consent withdrawn, lost to follow-up, noncompliance, never treated, missing data, protocol violation, death, and other reasons. Patients with HIV-1 RNA levels <400 copies/mL who chose not to continue in the voluntary extension phases of the study were censored at date of last dose of study medication. Respondera 69% 48% 57% 40% 50% 29% Virologic failureb 6% 12% 15% 20% 13% 19% Discontinued for adverse events 7% 8% 6% 8% 16% 20% Discontinued for other reasonsc 17% 31% 22% 32% 21% 32% CD4+ cell count (cells/mm3) Observed subjects (n) (279) (205) (256) (158) (228) (129) Mean change from
baseline190 329 191 319 180 329 For patients treated with SUSTIVA + zidovudine + lamivudine, SUSTIVA + indinavir, or indinavir + zidovudine + lamivudine, the percentage of responders with HIV-1 RNA <50 copies/mL was 65%, 50%, and 45%, respectively, through 48 weeks, and 43%, 31%, and 23%, respectively, through 168 weeks. A Kaplan-Meier analysis of time to loss of virologic response (HIV RNA <400 copies/mL) suggests that both the trends of virologic response and differences in response continue through 4 years.
ACTG 364 is a randomized, double-blind, placebo-controlled, 48-week study in NRTI-experienced patients who had completed two prior ACTG studies. One-hundred ninety-six patients (mean age 41 years [range 18-76], 74% Caucasian, 88% male) received NRTIs in combination with SUSTIVA (efavirenz) (600 mg once daily), or nelfinavir (NFV, 750 mg three times daily), or SUSTIVA (600 mg once daily) + nelfinavir in a randomized, double-blinded manner. The mean baseline CD4+ cell count was 389 cells/mm3 and mean baseline HIV-1 RNA level was 8130 copies/mL. Upon entry into the study, all patients were assigned a new open-label NRTI regimen, which was dependent on their previous NRTI treatment experience. There was no significant difference in the mean CD4+ cell count among treatment groups; the overall mean increase was approximately 100 cells at 48 weeks among patients who continued on study regimens. Treatment outcomes are shown in Table 11. Plasma HIV RNA levels were quantified with the AMPLICOR HIV-1 MONITOR assay using a lower limit of quantification of 500 copies/mL.
Table 11: Outcomes of Randomized Treatment Through 48 Weeks, Study ACTG 364* Outcome SUSTIVA + NFV +
NRTIs
(n=65)SUSTIVA + NRTIs
(n=65)NFV + NRTIs
(n=66)* For some patients, Week 56 data were used to confirm the status at Week 48. a Subjects achieved virologic response (two consecutive viral loads <500 copies/mL) and maintained it through Week 48. b Includes viral rebound and failure to achieve confirmed <500 copies/mL by Week 48. c See Adverse Reactions (6.1) for a safety profile of these regimens. d Includes loss to follow-up, consent withdrawn, noncompliance. HIV-1 RNA <500 copies/mLa 71% 63% 41% HIV-1 RNA ≥500 copies/mLb 17% 34% 54% CDC Category C Event 2% 0% 0% Discontinuations for adverse eventsc 3% 3% 5% Discontinuations for other reasonsd 8% 0% 0% A Kaplan-Meier analysis of time to treatment failure through 72 weeks demonstrates a longer duration of virologic suppression (HIV RNA <500 copies/mL) in the SUSTIVA-containing treatment arms.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Capsules
SUSTIVA® (efavirenz) capsules are available as follows:
Capsules 200 mg are gold color, reverse printed with “SUSTIVA” on the body and imprinted “200 mg” on the cap.
Capsules 50 mg are gold color and white, printed with “SUSTIVA” on the gold color cap and reverse printed “50 mg” on the white body.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
17.1 Drug Interactions
A statement to patients and healthcare providers is included on the product’s bottle labels:
ALERT: Find out about medicines that should NOT be taken with SUSTIVA.SUSTIVA may interact with some drugs; therefore, patients should be advised to report to their doctor the use of any other prescription, nonprescription medication, or herbal products, particularly St. John’s wort.
17.2 General Information for Patients
Patients should be informed that SUSTIVA is not a cure for HIV-1 infection and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. Patients should remain under the care of a physician while taking SUSTIVA.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breast-feed. It is not known if SUSTIVA can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breast-feed because HIV-1 can be passed to the baby in breast milk.
17.3 Dosing Instructions
Patients should be advised to take SUSTIVA every day as prescribed. SUSTIVA must always be used in combination with other antiretroviral drugs. Patients should be advised to take SUSTIVA on an empty stomach, preferably at bedtime. Taking SUSTIVA with food increases efavirenz concentrations and may increase the frequency of adverse reactions. Dosing at bedtime may improve the tolerability of nervous system symptoms [see Dosage and Administration (2) and Adverse Reactions (6.1)].
17.4 Nervous System Symptoms
Patients should be informed that central nervous system symptoms (NSS) including dizziness, insomnia, impaired concentration, drowsiness, and abnormal dreams are commonly reported during the first weeks of therapy with SUSTIVA [see Warnings and Precautions (5.5)]. Dosing at bedtime may improve the tolerability of these symptoms, which are likely to improve with continued therapy. Patients should be alerted to the potential for additive effects when SUSTIVA is used concomitantly with alcohol or psychoactive drugs. Patients should be instructed that if they experience NSS they should avoid potentially hazardous tasks such as driving or operating machinery.
17.5 Psychiatric Symptoms
Patients should be informed that serious psychiatric symptoms including severe depression, suicide attempts, aggressive behavior, delusions, paranoia, and psychosis-like symptoms have been reported in patients receiving SUSTIVA [see Warnings and Precautions (5.4)]. If they experience severe psychiatric adverse experiences they should seek immediate medical evaluation. Patients should be advised to inform their physician of any history of mental illness or substance abuse.
17.6 Rash
Patients should be informed that a common side effect is rash [see Warnings and Precautions (5.7)]. Rashes usually go away without any change in treatment. However, since rash may be serious, patients should be advised to contact their physician promptly if rash occurs.
17.7 Reproductive Risk Potential
Women receiving SUSTIVA should be instructed to avoid pregnancy [see Warnings and Precautions (5.6)]. A reliable form of barrier contraception must always be used in combination with other methods of contraception, including oral or other hormonal contraception. Because of the long half-life of efavirenz, use of adequate contraceptive measures for 12 weeks after discontinuation of SUSTIVA is recommended. Women should be advised to notify their physician if they become pregnant or plan to become pregnant while taking SUSTIVA. If this drug is used during the first trimester of pregnancy, or if the patient becomes pregnant while taking this drug, she should be apprised of the potential harm to the fetus.
17.8 Fat Redistribution
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known [see Warnings and Precautions (5.12)].
-
PATIENT PACKAGE INSERT
Patient Information
SUSTIVA® (sus-TEE-vah)
[efavirenz (eh-FAH-vih-rehnz)]
capsules and tablets
ALERT: Find out about medicines that should NOT be taken with SUSTIVA.
Please also read the section “MEDICINES YOU SHOULD NOT TAKE WITH SUSTIVA.”
Read this information before you start taking SUSTIVA. Read it again each time you refill your prescription, in case there is any new information. This leaflet provides a summary about SUSTIVA and does not include everything there is to know about your medicine. This information is not meant to take the place of talking with your doctor.
What is SUSTIVA?
SUSTIVA is a medicine used in combination with other medicines to help treat infection with Human Immunodeficiency Virus type 1 (HIV-1), the virus that causes AIDS (acquired immune deficiency syndrome). SUSTIVA is a type of anti-HIV drug called a “non-nucleoside reverse transcriptase inhibitor” (NNRTI). NNRTIs are not used in the treatment of Human Immunodeficiency Virus type 2 (HIV-2) infection.
SUSTIVA works by lowering the amount of HIV-1 in the blood (viral load). SUSTIVA must be taken with other anti-HIV medicines. When taken with other anti-HIV medicines, SUSTIVA has been shown to reduce viral load and increase the number of CD4+ cells, a type of immune cell in blood. SUSTIVA may not have these effects in every patient.
SUSTIVA does not cure HIV or AIDS and you may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. You should remain under the care of a doctor when using SUSTIVA.
Avoid doing things that can spread HIV-1 infection.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
What are the possible side effects of SUSTIVA?
Serious psychiatric problems. A small number of patients experience severe depression, strange thoughts, or angry behavior while taking SUSTIVA. Some patients have thoughts of suicide and a few have actually committed suicide. These problems tend to occur more often in patients who have had mental illness. Contact your doctor right away if you think you are having these psychiatric symptoms, so your doctor can decide if you should continue to take SUSTIVA (efavirenz).
Common side effects. Many patients have dizziness, trouble sleeping, drowsiness, trouble concentrating, and/or unusual dreams during treatment with SUSTIVA. These side effects may be reduced if you take SUSTIVA at bedtime on an empty stomach. They also tend to go away after you have taken the medicine for a few weeks. If you have these common side effects, such as dizziness, it does not mean that you will also have serious psychiatric problems, such as severe depression, strange thoughts, or angry behavior. Tell your doctor right away if any of these side effects continue or if they bother you. It is possible that these symptoms may be more severe if SUSTIVA is used with alcohol or mood altering (street) drugs.
If you are dizzy, have trouble concentrating, or are drowsy, avoid activities that may be dangerous, such as driving or operating machinery.
Rash is common. Rashes usually go away without any change in treatment. In a small number of patients, rash may be serious. If you develop a rash, call your doctor right away. Rash may be a serious problem in some children. Tell your child’s doctor right away if you notice rash or any other side effects while your child is taking SUSTIVA.
Other common side effects include tiredness, upset stomach, vomiting, and diarrhea. Some patients taking SUSTIVA have experienced increased levels of lipids (cholesterol and triglycerides) in the blood.
Changes in body fat. Changes in body fat develop in some patients taking anti-HIV medicine. These changes may include an increased amount of fat in the upper back and neck (“buffalo hump”), in the breasts, and around the trunk. Loss of fat from the legs, arms, and face may also happen. The cause and long-term health effects of these fat changes are not known.
Liver problems. Some patients taking SUSTIVA have experienced serious liver problems including liver failure resulting in transplantation or death. Most of these serious side effects occurred in patients with a chronic liver disease such as hepatitis infection, but there have also been a few reports in patients without any existing liver disease.
Tell your doctor or healthcare provider if you notice any side effects while taking SUSTIVA.
Contact your doctor before stopping SUSTIVA because of side effects or for any other reason.
This is not a complete list of side effects possible with SUSTIVA. Ask your doctor or pharmacist for a more complete list of side effects of SUSTIVA and all the medicines you will take.
How should I take SUSTIVA?
General Information
- You should take SUSTIVA on an empty stomach, preferably at bedtime.
- Swallow SUSTIVA with water.
- Taking SUSTIVA with food increases the amount of medicine in your body, which may increase the frequency of side effects.
- Taking SUSTIVA at bedtime may make some side effects less bothersome.
- SUSTIVA must be taken in combination with other anti-HIV medicines. If you take only SUSTIVA, the medicine may stop working.
- Do not miss a dose of SUSTIVA. If you forget to take SUSTIVA, take the missed dose right away, unless it is almost time for your next dose. Do not double the next dose. Carry on with your regular dosing schedule. If you need help in planning the best times to take your medicine, ask your doctor or pharmacist.
- Take the exact amount of SUSTIVA your doctor prescribes. Never change the dose on your own. Do not stop this medicine unless your doctor tells you to stop.
- If you believe you took more than the prescribed amount of SUSTIVA, contact your local Poison Control Center or emergency room right away.
- Tell your doctor if you start any new medicine or change how you take old ones. Your doses may need adjustment.
- When your SUSTIVA supply starts to run low, get more from your doctor or pharmacy. This is very important because the amount of virus in your blood may increase if the medicine is stopped for even a short time. The virus may develop resistance to SUSTIVA and become harder to treat.
- Your doctor may want to do blood tests to check for certain side effects while you take SUSTIVA (efavirenz).
Capsules
- The dose of SUSTIVA capsules for adults is 600 mg (three 200-mg capsules, taken together) once a day by mouth. The dose of SUSTIVA for children may be lower (see “Can children take SUSTIVA?”).
Tablets
- The dose of SUSTIVA tablets for adults is 600 mg (one tablet) once a day by mouth.
Can children take SUSTIVA?
Yes, children who are able to swallow capsules can take SUSTIVA. Rash may be a serious problem in some children. Tell your child’s doctor right away if you notice rash or any other side effects while your child is taking SUSTIVA. The dose of SUSTIVA for children may be lower than the dose for adults. Capsules containing lower doses of SUSTIVA are available. Your child’s doctor will determine the right dose based on your child’s weight.
Who should not take SUSTIVA?
Do not take SUSTIVA if you are allergic to the active ingredient, efavirenz, or to any of the inactive ingredients. Your doctor and pharmacist have a list of the inactive ingredients.
What should I avoid while taking SUSTIVA?
- Women should not become pregnant while taking SUSTIVA and for 12 weeks after stopping it. Serious birth defects have been seen in the offspring of animals and women treated with SUSTIVA during pregnancy. It is not known whether SUSTIVA caused these defects. Tell your doctor right away if you are pregnant. Also talk with your doctor if you want to become pregnant.
- Women should not rely only on hormone-based birth control, such as pills, injections, or implants, because SUSTIVA may make these contraceptives ineffective. Women must use a reliable form of barrier contraception, such as a condom or diaphragm, even if they also use other methods of birth control. SUSTIVA may remain in your blood for a time after therapy is stopped. Therefore, you should continue to use contraceptive measures for 12 weeks after you stop taking SUSTIVA.
- Do not breast-feed if you are taking SUSTIVA. It is not known if SUSTIVA can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breast-feed because HIV-1 can be passed to the baby in the breast milk. Talk with your doctor if you are breast-feeding. You may need to stop breast-feeding or use a different medicine.
- Taking SUSTIVA with alcohol or other medicines causing similar side effects as SUSTIVA, such as drowsiness, may increase those side effects.
- Do not take any other medicines without checking with your doctor. These medicines include prescription and nonprescription medicines and herbal products, especially St. John’s wort (Hypericum perforatum).
Before using SUSTIVA, tell your doctor if you
- have problems with your liver or have hepatitis. Your doctor may want to do tests to check your liver while you take SUSTIVA or may switch you to another medicine.
- have ever had mental illness or are using drugs or alcohol.
- have ever had seizures or are taking medicine for seizures [for example, Dilantin (phenytoin), Tegretol (carbamazepine), or phenobarbital]. Your doctor may want to switch you to another medicine or check drug levels in your blood from time to time.
What important information should I know about taking other medicines with SUSTIVA?
SUSTIVA may change the effect of other medicines, including ones for HIV, and cause serious side effects. Your doctor may change your other medicines or change their doses. Other medicines, including herbal products, may affect SUSTIVA. For this reason, it is very important to:
- let all your doctors and pharmacists know that you take SUSTIVA.
- tell your doctors and pharmacists about all medicines you take. This includes those you buy over-the-counter and herbal or natural remedies.
Bring all your prescription and nonprescription medicines as well as any herbal remedies that you are taking when you see a doctor, or make a list of their names, how much you take, and how often you take them. This will give your doctor a complete picture of the medicines you use. Then he or she can decide the best approach for your situation.
Taking SUSTIVA with St. John’s wort (Hypericum perforatum), an herbal product sold as a dietary supplement, or products containing St. John’s wort is not recommended. Talk with your doctor if you are taking or are planning to take St. John’s wort. Taking St. John’s wort may decrease SUSTIVA levels and lead to increased viral load and possible resistance to SUSTIVA or cross-resistance to other anti-HIV drugs.
MEDICINES YOU SHOULD NOT TAKE WITH SUSTIVA
The following medicines may cause serious and life-threatening side effects when taken with SUSTIVA. You should not take any of these medicines while taking SUSTIVA:
- Vascor (bepridil)
- Propulsid (cisapride)
- Versed (midazolam)
- Orap (pimozide)
- Halcion (triazolam)
- Ergot medications (for example, Wigraine and Cafergot)
The following medicine should not be taken with SUSTIVA since it contains efavirenz, the active ingredient in SUSTIVA:
- ATRIPLA (efavirenz, emtricitabine, tenofovir disoproxil fumarate)
The following medicines may need to be replaced with another medicine when taken with SUSTIVA:
- Fortovase, Invirase (saquinavir)
- Biaxin (clarithromycin)
- Carbatrol, Tegretol (carbamazepine)
- Noxafil (posaconazole)
- Sporanox (itraconazole)
- REYATAZ (atazanavir sulfate), if this is not the first time you are receiving treatment for your HIV infection
- Victrelis (boceprevir)
The following medicines may require a change in the dose of either SUSTIVA or the other medicine:
- Calcium channel blockers such as Cardizem or Tiazac (diltiazem), Covera HS or Isoptin SR (verapamil), and others.
- The cholesterol-lowering medicines Lipitor (atorvastatin), PRAVACHOL (pravastatin sodium), and Zocor (simvastatin).
- Crixivan (indinavir)
- Kaletra (lopinavir/ritonavir)
- Methadone
- Mycobutin (rifabutin)
- REYATAZ (atazanavir sulfate). If you are taking SUSTIVA and REYATAZ, you should also be taking Norvir (ritonavir).
- Rifadin (rifampin) or the rifampin-containing medicines Rifamate and Rifater.
- Selzentry (maraviroc)
- Vfend (voriconazole) and SUSTIVA must not be taken together at standard doses. Some doses of voriconazole can be taken at the same time as a lower dose of SUSTIVA, but you must check with your doctor first.
- Zoloft (sertraline)
- Wellbutrin, Wellbutrin SR, Wellbutrin XL, or Zyban (bupropion)
- The immunosuppressant medicines cyclosporine (Gengraf, Neoral, Sandimmune, and others), Prograf (tacrolimus), or Rapamune (sirolimus).
These are not all the medicines that may cause problems if you take SUSTIVA. Be sure to tell your doctor about all medicines that you take.
General advice about SUSTIVA:
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use SUSTIVA for a condition for which it was not prescribed. Do not give SUSTIVA to other people, even if they have the same symptoms you have. It may harm them.
Keep SUSTIVA at room temperature (77° F) in the bottle given to you by your pharmacist. The temperature can range from 59° to 86° F.
Keep SUSTIVA out of the reach of children.
This leaflet summarizes the most important information about SUSTIVA. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for the full prescribing information about SUSTIVA, or you can visit the SUSTIVA website at www.sustiva.com or call 1-800-321-1335.
__________________
SUSTIVA is a registered trademark of Bristol-Myers Squibb Pharma Company, ATRIPLA is a trademark of Bristol-Myers Squibb & Gilead Sciences, LLC, PRAVACHOL is a registered trademark of ER Squibb & Sons, LLC, and REYATAZ is a registered trademark of Bristol-Myers Squibb Company. Other brands listed are the trademarks of their respective owners.
Distributed by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USASUSTIVA® (efavirenz) capsules made in India.
© Bristol-Myers Squibb Company 2012
1262274A8
510230013IN09
Rev August 2012 -
PRINCIPAL DISPLAY PANEL
NDC 54868-4668-0 30 Tablets
SUSTIVA® Rx only
(efavirenz) tablets600 mg
Each tablet contains 600 mg of efavirenz.
DOSAGE: For dosage and full prescribing
information, read accompanying package
insert.
Note to pharmacist: Do not cover
ALERT box with pharmacy label.ALERT: Find out about medicines that
should NOT be taken with SUSTIVA®
-
INGREDIENTS AND APPEARANCE
SUSTIVA
efavirenz tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-4668(NDC:0056-0510) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength efavirenz (UNII: JE6H2O27P8) (efavirenz - UNII:JE6H2O27P8) efavirenz 600 mg Inactive Ingredients Ingredient Name Strength croscarmellose sodium (UNII: M28OL1HH48) hydroxypropyl cellulose (UNII: RFW2ET671P) lactose monohydrate (UNII: EWQ57Q8I5X) magnesium stearate (UNII: 70097M6I30) cellulose, microcrystalline (UNII: OP1R32D61U) sodium lauryl sulfate (UNII: 368GB5141J) carnauba wax (UNII: R12CBM0EIZ) Product Characteristics Color yellow (yellow) Score no score Shape OVAL (capsule-shaped) Size 19mm Flavor Imprint Code SUSTIVA;SUSTIVA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-4668-0 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021360 10/11/2012 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel(54868-4668)

