Label: CARTICEL- autologous cultured chondrocytes implant
-
Contains inactivated NDC Code(s)
NDC Code(s): 69866-1025-1 - Packager: Vericel Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 6, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Carticel® safely and effectively. See full prescribing information for Carticel.
Carticel (autologous cultured chondrocytes)
For Autologous Implantation
Initial U.S. Approval: 1997INDICATIONS AND USAGE
Carticel is an autologous cellular product indicated for the repair of symptomatic cartilage defects of the femoral condyle (medial, lateral or trochlea), caused by acute or repetitive trauma, in patients who have had an inadequate response to a prior arthroscopic or other surgical repair procedure (e.g., debridement, microfracture, drilling/abrasion arthroplasty, or osteochondral allograft/autograft). (1)
Carticel should be used only in conjunction with debridement, placement of a periosteal flap and rehabilitation. (1)
Carticel is not indicated for:
DOSAGE AND ADMINISTRATION
For Autologous Implantation Only
- Carticel should be administered only by physicians who have completed Vericel's Surgeon Training Program. (2.3)
- Implantation of the Carticel product is performed during arthrotomy and requires both preparation of the defect bed and a periosteal flap to secure the implant.
- See the Carticel Surgical Manual, Vericel document #65021 for instructions on the performance of these procedures. (2.3)
DOSAGE FORMS AND STRENGTHS
Each Carticel® vial of autologous cultured chondrocytes contains approximately 12 million cells per vial. (3)
CONTRAINDICATIONS
Do not use in patients with a known history of hypersensitivity to gentamicin, other aminoglycosides or materials of bovine origin. (4)
WARNINGS AND PRECAUTIONS
- The necessity of subsequent surgical procedures, primarily arthroscopic, following Carticel implantation is common. In the STAR study, 49% of patients underwent a subsequent surgical procedure, irrespective of relationship to Carticel. (5.1, 6.2)
- Carticel is not routinely tested for transmissible infectious diseases and may transmit diseases to the health care provider handling the product. Carticel is intended for autologous use only. (5.2)
- Pre-existing conditions, including meniscal tears, joint instability, or malalignment should be assessed and treated prior to or concurrent with Carticel implantation. (5.3)
- Carticel should not be used in patients who have previously had cancer in the bones, cartilage, fat or muscle of the treated limb. (5.4)
ADVERSE REACTIONS
The most common serious adverse events (> 5% of patients), derived from the STAR study include arthrofibrosis/joint adhesions, graft overgrowth, chondromalacia or chondrosis, cartilage injury, graft complication, meniscal lesion and graft delamination. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Vericel at 1-800-453-6948 or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
Use in children or patients over age 65 has not been assessed. (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2015
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Handling Precautions and Preparation
2.3 Administration
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Subsequent Surgical Procedures
5.2 Risk of Transmissible Infectious Diseases
5.3 Pre-surgical Assessment of Comorbidities
5.4 Product Safety and Sterility
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8. USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
8.5 Geriatric Use
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Pharmacology and/or Toxicology
14. CLINICAL STUDIES
14.1 Pre-Approval Studies
14.2 Post-Approval Studies
15. REFERENCES
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17. PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1. INDICATIONS AND USAGE
Carticel® is indicated for the repair of symptomatic cartilage defects of the femoral condyle (medial, lateral or trochlea), caused by acute or repetitive trauma, in patients who have had an inadequate response to a prior arthroscopic or other surgical repair procedure (e.g., debridement, microfracture, drilling/abrasion arthroplasty, or osteochondral allograft/autograft).
Carticel should be used only in conjunction with debridement, placement of a periosteal flap and rehabilitation. The independent contributions of the autologous cultured chondrocytes and other components of the therapy to outcome are unknown.
Carticel is not indicated for the treatment of cartilage damage associated with generalized osteoarthritis.
Carticel is not recommended for patients with total meniscectomy unless surgically reconstructed prior to or concurrent with Carticel implantation.
-
2. DOSAGE AND ADMINISTRATION
For Autologous Implantation Only
2.1 Dosage
Patients in the Swedish series [see Clinical Studies (14)] received a wide range of cell doses per cm2 of defect. Available data on 70 of 78 patients with femoral condyle defects showed a median dose of 1.6 million cells/cm2 of defect. The middle 80% of these patients received from 0.64 million to 3.3 million cells/cm2.
Each Carticel finished product vial contains approximately 12 million cells. Vericel provides a single vial for each defect measuring ≤ 7 cm2. Two vials of Carticel are provided for defects 7 to 14 cm2, and three vials are provided for defects > 14 cm2. This is based on Vericel's greater than 10 years of experience with Carticel.
2.2 Handling Precautions and Preparation
2.2.1 Handling Precautions
The Carticel product is intended solely for autologous use. Prior to Carticel implantation, match the patient name and ID number on the certificate of analysis to the patient's chart and the patient ID on the shipping box, transport cylinder and vial.
Health care providers should employ universal precautions in handling the biopsy samples and the Carticel product [see Risk of Transmissible Infectious Diseases (5.2)].
Refer to the Indications and Usage (1) and Warnings and Precautions (5) Sections for additional considerations regarding the use of Carticel.
2.2.2 Preparation
NOTE:
The exterior of the Carticel vial containing the cultured cells is NOT sterile. Follow strict sterile technique protocols.
When treating a defect that requires multiple vials of cells, resuspend, aspirate and inject one vial at a time.
- Remove red plastic lid from vial. Wipe the vial surface and lid with alcohol.
- Inspect vial contents for particulates, discoloration or turbidity. The cellular product appears as a yellowish clump in the bottom of the vial. Do not administer if contents appear turbid prior to cell suspension.
- While holding vial in a vertical position, insert the needle of the intraspinal catheter into the vial. The needle must be positioned just above the fluid level. Slowly remove the inner needle from the catheter, leaving flexible tip behind. Attach a tuberculin syringe to catheter.
- Lower the catheter tip into the media and position just above the cell pellet. Aspirate all the medium from the vial leaving only the cell pellet behind. Slowly expel medium back into the vial. This action will break the cell pellet and resuspend the cells in the medium.
- Lower the catheter tip to the base of the vial and aspirate all contents into syringe, leaving the vial empty. Slowly inject the contents into the vial again. This will assure complete suspension of the cells. Repeat these steps as needed to ensure all cells are resuspended. Cell resuspension is complete when cell particles are no longer apparent, and the medium is a consistent, "cloudy" mixture. Aspirate all contents of vial into syringe. Always hold syringe vertical to keep an air pocket at the proximal end of syringe.
2.3 Administration
Implantation of the Carticel product should be restricted to physicians who have completed Vericel's Surgeon Training Program.
Implantation of the Carticel® product is performed during arthrotomy and requires both preparation of the defect bed and a periosteal flap to secure the implant. Complete hemostasis must be achieved prior to periosteal fixation and cell implantation. See the Carticel Surgical Manual, Vericel document #65021 for instructions on the performance of these procedures.
2.3.1 Implantation
- Insert the catheter tip through the superior opening of the periosteal chamber at the site of the defect. Advance catheter to most inferior aspect of the defect.
- Slowly inject a cell dose while moving the catheter tip from side to side and withdrawing the catheter proximally. This will ensure an even distribution of the cells throughout the defect.
- Complete the implantation by closing the superior opening of the periosteum as instructed. See Carticel Surgical Manual, Vericel document #65021.
- 3. DOSAGE FORMS AND STRENGTHS
-
4. CONTRAINDICATIONS
Carticel should not be used in patients with a known history of hypersensitivity to gentamicin, other aminoglycosides or materials of bovine origin.
Gentamicin is added to both the cartilage biopsy transport media and in the culture media used during the processing of Carticel. Residual quantities of gentamicin up to 5 µg/mL are present in the Carticel product.
Fetal bovine serum is a component in the culture medium used to propagate the autologous chondrocytes. Trace quantities of bovine-derived proteins may be present in the Carticel product.
-
5. WARNINGS AND PRECAUTIONS
5.1 Subsequent Surgical Procedures
Review of the data from the Study of the Treatment of Articular Repair (STAR) and the Registry Based Study (RBS) [see Clinical Trials Experience (6.1)] as well as the Carticel worldwide experience (adverse reactions solicited through the Cartilage Repair Registry (CRR) and spontaneous reports) as of November 21, 1997 showed that the occurrence of a subsequent surgical procedure, primarily arthroscopic, following Carticel implantation is common. In the STAR study, 49% of patients underwent a subsequent surgical procedure, irrespective of relationship to Carticel (6.2). Symptoms leading to arthroscopic intervention included catching, locking, clicking or pain. Patients who develop clinical signs of tissue hypertrophy, including catching or clicking, should be evaluated and arthroscopy may be indicated for treatment or further assessment.
5.2 Risk of Transmissible Infectious Diseases
The Carticel product is intended solely for autologous use. Patients undergoing the surgical procedures associated with Carticel are not routinely tested for transmissible infectious diseases. Therefore, the cartilage biopsy and the Carticel product may carry the risk of transmitting infectious diseases to the health care provider handling these tissues. Accordingly, health care providers should employ universal precautions in handling the biopsy samples and the Carticel product.
5.3 Pre-surgical Assessment of Comorbidities
The following conditions should be assessed and treated prior to or concurrent with implantation with Carticel:
- Unstable meniscus tears should be repaired or resected.
- If the patient has had a total meniscectomy, absent meniscus should be reconstructed.
- Instability of the knee may adversely affect the success of the procedure and should be corrected. The anterior and posterior cruciate ligaments should be free of laxity as well as stable and intact. It is recommended that cruciate deficiencies be corrected.
- Abnormal weight-distribution within the joint may adversely affect the success of the procedure and should be corrected. The tibial/femoral joint should be properly aligned. When treating trochlear defects, abnormal patellar mechanics should be assessed and corrected.
5.4 Product Safety and Sterility
The safety of the Carticel product is unknown in patients with malignancy in the area of cartilage biopsy or implant. The potential exists for in vitro expansion and subsequent implantation of malignant or dysplastic cells present in biopsy tissue. In addition, implantation of normal autologous chondrocytes could theoretically stimulate growth of malignant cells in the area of the implant, although there have been no reported incidents in humans or animals.
The Carticel product is shipped following a preliminary sterility test with a 48-hour incubation to determine absence of microbial growth. Final (14 day incubation) sterility test results are not available at the time of implantation.
-
6. ADVERSE REACTIONS
Information on the safety of implanted autologous chondrocytes is derived from the Study of the Treatment of Articular Repair (STAR) [see Clinical Studies (14)], the Cartilage Repair Registry, the Swedish Series, and post-marketing adverse event reporting.
The most common serious adverse events (> 5% of patients) derived from the STAR study include arthrofibrosis/joint adhesion, graft overgrowth, chondromalacia or chondrosis, cartilage injury, graft complication, meniscal lesion and graft delamination. Only serious adverse events were collected in this study.
6.1 Clinical Trials Experience
The adverse reaction rates as well as the rate and type of subsequent surgical procedures from Carticel® studies of different designs cannot be directly compared amongst each other. Adverse reaction data from these studies do, however, provide a basis for identifying adverse reactions that may be related to product use and for estimating their frequency.
Study of the Treatment of Articular Repair (STAR)
In the STAR study [see Study of the Treatment of Articular Repair (STAR) (14.2.2)], patients who had experienced an inadequate response to a prior cartilage repair procedure underwent Carticel implantation to the index lesion. A total of 154 patients were implanted with Carticel; 28 patients discontinued the study early. The numbers of patients completing the 24 and 48 month follow-up visits are 136 and 115, respectively. Mean patient age was 35 years at consent. The majority of patients were Caucasian (135; 88%) and male (106; 69%).
Seventy-six (76) (49% of 154) patients underwent 113 subsequent surgical procedures (SSPs) on the treated knee, irrespective of relationship to Carticel, during the 4 year follow-up. Of the 76 patients, 52 patients had 1 SSP, 15 patients had 2 SSPs, and 9 patients had 3 or more SSPs. Sixty-one (61) (80%) of the 76 patients who had an SSP underwent a procedure within the first 24 months after implantation. The majority of patients, 83% (63 of the 76), underwent an arthroscopy or manipulation under anesthesia only. Table 1 shows the interventions during SSPs in > 2% of patients.
Table 1: Interventions during Subsequent Surgical Procedures, Regardless of Relationship, in > 2% of Patients Intervention % of 154 Patients Debridement of Cartilage Lesion* 31% (47/154) Lysis of Adhesions 14% (21/154) Synovectomy / Synovial Plica Excision 12% (19/154) Other Debridement† 10% (16/154) Chondroplasty 6% (10/154) Meniscectomy 6% (10/154) Loose Body Removal 5% (7/154) Microfracture – Index Lesion 5% (7/154) Scar Tissue Removal 5% (7/154) Release of Patellar Retinaculum 4% (6/154) Hardware Removal 4% (6/154) Microfracture – New Lesion 4% (6/154) Osteotomy 3% (5/154) Lysis of adhesions was the most frequent surgical intervention performed in the first 6 months. After 6 months, cartilage debridement was the most frequently performed intervention. In the STAR study, 61% (46/76) of patients who required an SSP after Carticel did not meet failure criteria by either modified Cincinnati score or surgical criteria (e.g., graft delamination or surgical procedure violating the subchondral bone).
The most clinically significant interventions or findings involving the Carticel graft or periosteal patch are as follows: 3 Carticel grafts were completely removed and 1 was partially removed due to delamination. Partial delamination or fraying of the graft or periosteal patch was reported in 10 additional patients. Four (4) of these patients underwent reattachment/repair of the periosteal patch. Finally, a partially intact graft was found in 1 patient who was re-implanted. Detailed lists of interventions and findings that may have been associated with the graft or periosteal patch are presented in Tables 1 and 2, respectively.
Table 2 shows the serious adverse events (SAEs) that occurred in ≥ 5% of patients, regardless of relationship to study treatment.
Table 2: Most Frequent Serious Adverse Events (in ≥ 5% of Patients), Regardless of Relationship, in the STAR Study Serious Adverse Events % of 154 Patients Arthrofibrosis/Joint Adhesions 16% (25/154) Graft Overgrowth 15% (23/154) Chondromalacia or Chondrosis 12% (18/154) Cartilage Injury* 11% (17/154) Graft Complication† 10% (15/154) Meniscal Lesion 8% (12/154) Graft Delamination 6% (9/154) Osteoarthritis 5% (7/154) Registry Based Study (RBS)
Data from a cohort of 97 Carticel® treated patients, who were retrospectively evaluated in the Registry Based Study [see Registry-Based Study (RBS) (14.2.1)], showed that 39% (38/97) of patients had a SSP within 3 years of which 63% (24/38) were assessed as related to Carticel. Shaving or trimming (debridement) of overgrown tissue (hypertrophic) commonly relieved patients' symptoms. In the RBS, 67% (16/24) of patients who required arthroscopy after Carticel had a good clinical benefit in terms of improved function and relief of symptoms. Table 3 shows the findings at surgery for the 38 patients who underwent a surgical procedure after Carticel.
Table 3: Most Frequent Findings (in ≥ 5% of Patients) at Subsequent Surgical Procedures in the Registry Based Study Symptoms or Surgical Findings
(MedDRA preferred term)% of 97 Patients Graft Overgrowth 10% (10/97) Partial Graft Delamination 8% (8/97) Chondromalacia 8% (8/97) Arthrofibrosis/Joint Adhesions 8% (8/97) Arthralgia 7% (7/97) Synovitis 6% (6/97) Meniscal Lesion 5% (5/97) Loose Body 5% (5/97) Swedish Series
Of 153 patients treated with autologous cultured chondrocyte implantation in the Swedish Series [see Clinical Studies (14)], 22% (34/153) of patients experienced the adverse reactions presented in Table 4 below.
Table 4: Initial ACI Experience Swedish Series Serious Adverse Reactions (Occurring at a frequency of 1% or more) Serious Adverse Reactions % of 153 Patients Tissue Hypertrophy See below Intra-articular Adhesions 8% Superficial Wound Infection 3% Hypertrophic Synovitis 3% Post-operative Hematoma 2% Adhesions of the Bursa Suprapatellaris 2% Hypertrophic Synovium 1% About 1% of patients developed severe adhesions resulting in "frozen knee" and requiring lysis. Adverse reactions noted at a level of less than 1% included keloid-like scar, pannus formation, significant swelling of the joint, pain with post-operative fever, and hematoma following arthroscopy.
In this series, arthroscopy was scheduled to be undertaken at 18 months of follow-up, regardless of patient symptoms. Of the patients who had arthroscopy, 43% (37/86) had hypertrophic tissue.
Forty of the 85 patients had femoral condyle defects. Of these, 25% (10/40) of patients had some hypertrophic tissue noted at follow-up arthroscopy. Some patients had clinical symptoms that included painful crepitations or catching, and these symptoms generally resolved after arthroscopic resection of the hypertrophic tissue. Ten percent (10%) of patients with hypertrophy required additional treatment after hypertrophic tissue recurred following initial resection. Not all patients with tissue hypertrophy noted at arthroscopy were symptomatic.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Carticel®. Most of these reactions are reported voluntarily, and it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Furthermore, the reported frequency of spontaneous reports underestimates the true frequency of adverse reactions. As of July 31, 2006, approximately 12,500 patients have been implanted with Carticel and 559 patients have reported serious adverse reactions after treatment. The most frequently identified operative findings in these patients, in descending order of frequency, were graft overgrowth, graft delamination (partial or complete), arthrofibrosis, joint adhesions, meniscus lesion or tear, graft complications, chondromalacia, loose body in knee joint, and joint malalignment.
- 8. USE IN SPECIFIC POPULATIONS
-
11. DESCRIPTION
Autologous cultured chondrocytes, the Carticel product, are derived from in vitro expansion of chondrocytes harvested from the patient's normal, femoral articular cartilage. Biopsies from a lesser-weight bearing area are the source of chondrocytes, which are isolated, expanded through cell culture, and implanted into articular cartilage defects beneath an autologous periosteal flap. Prior to final packaging, cell viability is assessed to be at least 80%.
Each single use container of autologous cultured chondrocytes has approximately 12 million cells aseptically processed and suspended in 0.4 mL of sterile, buffered Dulbecco's Modified Eagles Medium (DMEM). Both the biopsy transport media and the cell culture media contain gentamicin. Residual quantities of gentamicin up to 5 µg/mL may be present in the Carticel product.
-
12. CLINICAL PHARMACOLOGY
Hyaline cartilage forms the articular surface of the femoral condyle. Studies have shown that implantation of autologous chondrocytes into the articular defect can result in the development of hyaline-like cartilage [see Clinical Studies (14)].1,2,3,4,5 Normal hyaline cartilage consists of chondrocytes (≤ 5% total volume) and extracellular matrix (≥ 95% total volume). The matrix contains a variety of macromolecules, including type II collagen and proteoglycan. The structure of the matrix allows the hyaline cartilage to absorb shock and withstand shearing and compression forces. Normal hyaline cartilage also has an extremely low coefficient of friction at the articular surface. Damage to articular cartilage from acute or repetitive trauma often results in pain and disability. However, because hyaline cartilage is avascular, spontaneous healing of large defects is not believed to occur in humans.
A variety of surgical procedures have been used in attempts to promote repair of articular cartilage, and a few studies have evaluated the histology resulting from these interventions. Generally, procedures such as marrow stimulation techniques (MST) have been shown to produce fibrocartilage or hybrid mixtures of fibrocartilage and hyaline cartilage. Published data show that autologous chondrocyte implantation (ACI) is more likely than MST to result in hyaline-like cartilage at the repair site. 1,2,4,5 However, because of differences in study design, lesion size and concomitant patient conditions, these data are not sufficient to draw conclusions concerning the long-term correlation of histology and clinical outcomes.
-
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or mutagenicity data are available for Carticel in animals or in humans. No studies on the effects of Carticel on fertility have been conducted.
13.2 Animal Pharmacology and/or Toxicology
Pre-Approval Studies
Bioactivity of autologous chondrocytes implanted under a periosteal patch was reported in the BLA for three rabbit studies6,7,8 of up to 52 weeks duration post-implant and one dog study9 of up to 18 months duration post-implant.
Rabbit Studies
- Histologic evaluations were performed at 8, 12 and 52 weeks. Improved healing of experimental defects implanted with autologous chondrocytes was observed compared to periosteal flap alone at 8, 12 and 52 weeks.
Dog Study
- Histologic evaluations were performed at 6 and 12 weeks and 12 and 18 months. Autologous chondrocytes showed improved healing compared to both empty defects and to defects covered with periosteum alone at 6 and 12 weeks. However, by the 12 and 18 month evaluations, the repair tissue had deteriorated so that no advantage of ACI over periosteum alone controls was demonstrated.
Beyond histologic variability of the defect site, no adverse tissue reaction was identified in any animals in these studies.
Post-Approval Studies
Five additional large animal, post-approval studies were performed.
Goat Studies
- Three of four goat studies were 16 weeks in duration. In the fourth study, the goats were sacrificed immediately after periosteal membrane placement. Despite difficulty in post-operative management of goats and resulting subchondral plate collapse in some animals in the 16-week studies, results from all four studies suggested that chondrocytes may contribute to histological repair of focal cartilage lesions. In the only bilateral joint study, serious subchondral collapse and uniformly poor repair resulted in inconclusive data. No safety issues were identified in any of these studies.
Horse Study
- The 8-week study included two experimental arms in order to model repair of cartilage lesions with or without subchondral penetration. Both models exhibited destruction/dislodgement of the periosteal flap; however, results suggested that chondrocytes may contribute to histologic repair in cartilage defects with subchondral penetration.
While the defects in all animal models exhibited highly variable repair tissue quality (resulting in only moderate histologic scores) the best repairs with implanted chondrocytes produced hyaline-like cartilage characterized by matrix predominating in type II collagen and saffranin-O or toluidine blue staining proteoglycan. Chondrocyte labeling in one of the rabbit studies8 and in an independent study in goats by Dell'Accio et al10 demonstrated that the hyaline-like matrix in these defects was the product of the implanted autologous chondrocytes.
-
14. CLINICAL STUDIES
14.1 Pre-Approval Studies
Clinical information regarding the use of autologous cultured chondrocytes was obtained from 2 open-label, observational studies consisting of a series of patients treated in Sweden and the Cartilage Repair Registry. Patients in the Swedish series received an autologous cultured chondrocyte product similar to Carticel®.
14.1.1 Swedish Series
The series consists of 153 patients who received autologous chondrocyte implantation for various defects of the knee. Patients presented with cartilage defects of the femoral condyle, patella, tibia, a combination of these, or osteochondritis dissecans, with or without comorbidity such as anterior cruciate ligament insufficiency requiring reconstruction.
Following autologous chondrocyte implantation, patients were followed for various durations. Clinical follow-up ranged from 1 week to 94 months; 86 patients had at least 18 months of follow-up. Most patients had arthroscopic evaluation; a subset had biopsy and histological evaluations. All patients were retrospectively classified as having one of three clinical outcomes: resumed all activities, some improvement, or no improvement. Clinical outcomes were also reported for patient subgroups including: 1) 40 patients with femoral condyle lesions, 2) 12 patients with osteochondritis dissecans lesions and 3) 22 patients who failed a prior debridement.
1) Clinical Outcome - Patients with Femoral Condyle Lesions
A total of 78 of 153 patients had femoral condyle lesions with or without co-morbidity. Patients had one or more defects ranging in size from < 1-20 cm2. Of the patients with femoral condyle lesions, 40 were evaluable after at least 18 months (median = 25; range = 18 to 94 months). Clinical outcomes for the 40 patients are summarized in Table 5.
Table 5: Patient Response to Treatment Defect Resumed All Activities Some Improvement No Improvement Total Patients Femoral Condyle 7
(29%)8
(33%)9
(38%)24 Femoral Condyle plus other
Non-Cartilage Repair4
(25%)9
(56%)3
(19%)16 Total 11
(28%)17
(42%)12
(30%)40 2) Clinical Outcome – Patients with Osteochondritis Dissecans Lesions
Of the 12 patients who received autologous cultured chondrocytes for treatment of an osteochondritis lesion, 6 of the 12 had "resumed all activities", 4 had "some improvement" and 2 had "no improvement" after the 18-month (median = 25; range = 18-94 months) follow-up period.
3) Clinical Outcome – Failed Earlier Procedures
Debridement of the cartilage defect is often performed along with administration of autologous cultured chondrocytes. To help differentiate the effects of the autologous cultured chondrocyte implantation procedure from those of debridement alone, an analysis was performed on 22 patients who had failed prior debridement and had a follow-up period after autologous cultured chondrocyte implantation which was at least as long as the time period to failure of their initial debridement. At the end of follow-up, 5 of the 22 patients had a functional outcome rating of "resumed all activities", 8 of the 22 patients had a rating of "some improvement" and 9 of the 22 patients had a rating of "no improvement." Thus, 13 of the 22 patients (59%) who had failed an earlier debridement had outcomes that were more favorable and durable following autologous cultured chondrocyte implantation than their previous debridement without cells.
Histological Outcome
Twenty-two (22) patients in the Swedish series had histological evaluation of biopsies from the implant site one or more years after their autologous chondrocyte implantation. Fifteen (15) of those patients had defects of the femoral condyle and 7 had defects of the patella. Six (6) of the 15 femoral condyle biopsies showed hyaline-like cartilage, 5 had a mixture of hyaline and fibrocartilage, and 4 had only fibrocartilage. Of the 6 biopsies with hyaline-like cartilage, 2 had minimal to no surface irregularities and 4 had some surface irregularities (e.g., fissures, fibrillations, etc.).
Arthroscopic Outcome
As an objective outcome evaluation, 86 of the 153 patients had a follow-up arthroscopy for investigational purposes at 18 months or more post-implantation. In some cases, the quality of repair observed at arthroscopy was considered to be supportive of the clinical or functional outcomes. A substantial number of patients were noted at arthroscopy to have tissue hypertrophy [see Adverse Reactions (6)].
14.1.2 Cartilage Repair Registry
The Cartilage Repair Registry (CRR) was established upon the introduction of Carticel® into orthopedic practice in March of 1995. The CRR was designed to prospectively collect the clinical outcomes of Carticel and other cartilage repair treatments for chondral lesions in the knee. Clinical data were collected at baseline arthroscopy, implantation, intervals of 6 and 12 months, and annually thereafter; adverse reaction data were collected on an ongoing basis through CRR adverse reaction collection and spontaneous reporting. Inclusion in the CRR was based on a qualifying event that was defined as a knee arthroscopy in which a chondral lesion was identified and a cartilage biopsy was harvested. Participation in the CRR was voluntary, and not all patients biopsied or implanted were included. As of November 21, 1997, 891 patients had been implanted worldwide, and 644 of these patients were included in the CRR. Functional outcomes were based on responses to a modified version of the Cincinnati Knee Rating System.
Data from a subset of 191 US patients in the CRR as of December 31, 1996 who had undergone repair of lesions on the femoral condyle (medial, lateral or trochlea) were assessed to support licensure. Patients were between the ages of 15-57, 66% (126/191) were male, and 34% (64/191) were female and one patient's gender was not reported. Of these 191 patients, 38 had at least 12 months of follow-up. At study baseline, these 38 patients' mean rating of overall condition was 3.2, which is defined as fair to poor: limitations that affect activities of daily living-no sports possible. At 12 month follow-up, these patients reported an overall condition score of 6.4 defined as good: some limitation with sports but can participate if patient compensates. Although these patients were rated according to outcome measurements different from those used in the Swedish series, the results were consistent with the Swedish experience.
14.2 Post-Approval Studies
Two post-approval studies were conducted and completed as a condition of approval for Carticel®: the Registry Based Study (RBS) and the Study of the Treatment of Articular Repair (STAR).
14.2.1 Registry-Based Study (RBS)
The RBS was a retrospective analysis of data collected for a cohort of 97 US patients treated between March of 1995 and March of 1997. Of the 97 patients enrolled, 95% completed 1-year follow-up, 80% completed 2-year follow-up and 74% completed 3-year follow-up. Of these 97 patients, 44 were part of the subset of 191 US patients in the CRR described above. A limitation of this study is the lack of a control group. Patients included in this study had a prior non-Carticel cartilage repair procedure (e.g., debridement or marrow stimulation procedure) performed at the time of the index arthroscopy, subsequently failed this procedure and went on to receive Carticel. In the 5 years prior to the index arthroscopy for the study, this patient population had received prior knee surgeries to include: 47% (46/97) of patients had at least one debridement/lavage of a cartilage defect, 25% (24/97) of patients had a bone marrow stimulation procedure, 31% (30/97) had at least one diagnostic arthroscopy, 30% (29/97) had at least one meniscus repair/meniscectomy and 10% (10/97) of patients had a ligament repair/reconstruction performed on the treated knee.
Using a modified Cincinnati Knee Rating System at study baseline, this patient population had a mean overall condition score of 3.1 defined as fair to poor: limitations that affect activities of daily living-no sports possible. Patients included were between the ages of 16-56, 69% (67/97) were male and 31% (30/97) were female. For the type of defect, 62% (60/97) of the defects were acute while 37% (36/97) were chronic. Of the treated defects, 75% (73/97) were treated on the medial femoral condyle (MFC), 26% (25/97) on the lateral femoral condyle (LFC) and 19% (18/97) on the trochlea. Adverse reactions collected during this study are provided in Adverse Reactions (6).
14.2.2 Study of the Treatment of Articular Repair (STAR)
The STAR study was an open-label within patient comparison of a prior non-Carticel (index) procedure to implantation of Carticel for articular cartilage defects of the distal femur. All patients had experienced an inadequate response to a prior non-Carticel surgical treatment, defined as both: a) patient and surgeon agreement that the patient's symptoms/function required surgical re-treatment of the defect and b) the patient's rating of the overall condition of the knee was a score ≤ 5 using the Modified Cincinnati Knee Rating System. In this patient population, the median time to meet the failure criteria was 3.4 months for the prior non-Carticel procedure and 90% of patients failed within 10.3 months. Patients who met these criteria were treated with Carticel and assessed every 6 months for up to 4 years.
Treatment failure for Carticel was defined as any of the following: a) the patient underwent surgical retreatment that violated the subchondral bone or reimplantation with Carticel for the same index defect, b) complete delamination or removal of the graft, or c) the patient's rating of the overall condition of the knee using the Modified Cincinnati Knee Rating System failed to improve from the baseline knee score over 3 consecutive 6-month intervals.
A total of 154 patients were treated with Carticel. At the index surgery required for study entry, patients had one or more of the following interventions: 120 patients (78%) had debridement, 44 patients (29%) had microfracture, 18 (12%) had subchondral drilling, 10 (6%) had abrasion arthroplasty, and 7 (5%) had an osteochondral autograft. The mean lesion size was 4.6 (± 3.2, SD) cm2. Fifty patients (32%) had multiple lesions in the reference knee and 29 patients had Carticel implanted in more than one lesion. Lesions that were implanted were located on the medial femoral condyle in 109 patients, lateral femoral condyle in 32 patients and trochlea in 46 patients. Forty patients (26%) had lesions which involved osteochondritis dissecans (OCD).
Of the 154 patients treated with Carticel, 28 patients discontinued the study early. The numbers of patients completing the 24 and 48 month follow-up visits are 136 and 115, respectively. The majority of Carticel patients (N= 117) did not meet failure criteria during the study. By the end of the study, a total of 37 patients met the treatment failure criteria. Results for the 40 patients with OCD lesions were comparable to the total study population as 34 (85%) did not meet the failure criteria for the study and 6 (15%) failed treatment with Carticel. Table 6 illustrates, during each year of follow-up, the number of patients who failed Carticel by the surgical criteria along with the number of patients who failed by the Overall Modified Cincinnati scale criteria.
Table 6: Category and Timing of Treatment Failure for Patients who Met Treatment Failure Criteria (N=37) 1 Year 2 Years 3 Years 4 Years Total Patients who Failed During the Interval 14 11 11 1 37 Surgery criteria 0 5 5 1 11 Overall Modified Cincinnati Scale criteria 14 6 6 0 26 The Overall Modified Cincinnati mean baseline score for the patient population as a whole was 3.26, poor: significant limitations that affect activities of daily living, to fair: moderate limitations that affect activities of daily living, no sports possible. At 48 months, the mean score was 6.39, good: some limitations with sports but can participate/compensate. The improvement was statistically significant. Table 7 shows the improvement in the Overall Modified Cincinnati score over time.
Table 7: Mean Overall Modified Cincinnati Score at Baseline and Follow-up Visits Visit Baseline
N=154Month 12
N=146Month 24
N=131Month 36
N=104Month 48
N=101- *
- Scores are for patients who returned for follow-up. Patients who failed by score criteria are included and patients who failed by surgical criteria are excluded from the scores for timepoints after the failure criteria were met.
Overall Modified Cincinnati Score*
Mean (SD)3.26
(1.02)
5.58
(1.99)
5.92
(2.08)
5.87
(2.19)
6.39
(2.31)
In addition to the change over time in activity level as measured with the Overall Modified Cincinnati Scale, there were similar and consistent changes in knee symptoms and function as measured with the Knee Injury and Osteoarthritis Outcome Score (KOOS), a measure of knee-specific symptoms and function consisting of the following five subscales: pain, symptoms, sports and recreation, knee-related quality of life, and activities of daily living. At 12 months post-Carticel® implant, the mean improvement from baseline for the patient population as a whole in each subscale was as follows: pain 19 (N = 146), symptoms 15 (N = 147), sports and recreation 17 (N = 129), knee-related quality of life 18 (N = 147), and activities of daily living 18 (N = 145). At 48 months post-Carticel implant, the mean improvement from baseline was as follows: pain 24 (N = 100), symptoms 19 (N = 101), sports and recreation 31 (N = 86), knee-related quality of life 32 (N = 101), and activities of daily living 23 (N = 99).
Adverse reactions collected during this study are provided in Adverse Reactions (6).
-
15. REFERENCES
- Knutsen G, Engebretsen L, Ludvigsen T, et al. Autologous chondrocyte implantation compared with microfracture in the knee. J Bone Joint Surg, 2004; 86A:455-464.
- Bentley G, Biant LC, Carrington RWJ, et al. A prospective, randomized comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85-B:223-230.
- Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895.
- Peterson L, Minas T, Brittberg M, et al. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop. 2000;1(374):212-234.
- Peterson L, Brittberg M, Kiviranta I, et al. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12.
- Peterson L, Menche D, Grande D, et al. Chondrocyte transplantation-an experimental model in the rabbit. Transactions from the 30th Annual Orthopedic Research Society, Atlanta, February 7-9, 1984. Palatine, III.:Orthopedic Research Society, 1984:218. Abstract.
- Grande DA, Pitman MI, Peterson L, et al. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7(2):208-18.
- Brittberg M, Nilsson A, Lindahl A, et al. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res. 1996 May;(326):270-83.
- Breinan H, Minas T, Barone L, et al. Histological evaluation of the course of healing of canine articular cartilage defects treated with cultured autologous chondrocytes. Tiss Engin 1998; 4(1):101-14.
- Dell'Accio F, Vanlauwe J, Bellemans, et al. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. Journal of Orthopaedic Research 21 2003; 21: 123–131.
-
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
The Carticel product, NDC 69866-1025-1, consists of viable, autologous cells packaged and labeled for implantation within specified time limits. Each vial contains approximately 12 million autologous cells for a single implantation procedure.
The shipping vials containing chondrocytes are accompanied by a technical data sheet with detailed specifications for the processed cells. The vial(s) of cells is placed within secondary packaging capable of maintaining the appropriate storage temperature and cell viability for up to 72 hours.
16.2 Storage and Handling
The Carticel® transport box should be held at room temperature and remain closed until the time of implantation to ensure proper storage conditions for the cells.
Do Not Refrigerate, Freeze, or Incubate the Carticel Shipping Container or its Contents.
Do Not Sterilize.
If the Vial is Damaged or Sterility has been Compromised, Do Not Use.
-
17. PATIENT COUNSELING INFORMATION
Patients receiving autologous cultured chondrocytes for treatment of an articular cartilage defect should receive the following information and instructions.
- Physical activity should be resumed according to the rehabilitation plan recommended by the physician. Generally, protected weight bearing is recommended for the first 6 to 8 weeks following implantation. The patient should receive specific instructions on crutch use, ambulation and weight bearing advancement on the treated limb.
- If pain starts to develop as the next level of activity is increased, decrease activity to the former level until the pain resolves.
- If exercise causes pain and/or swelling, reduce the amount of physical activity.
- Swelling should be controlled using ice packs.
- Patient adherence to the prescribed rehabilitation program is important and activity at variance from the rehabilitation program may compromise clinical benefit from Carticel.
- At anytime during the rehabilitation process or after, if sharp pain with locking or swelling is experienced, contact the physician for medical advice.
- SPL UNCLASSIFIED SECTION
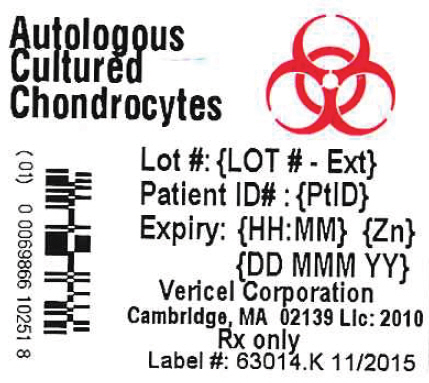
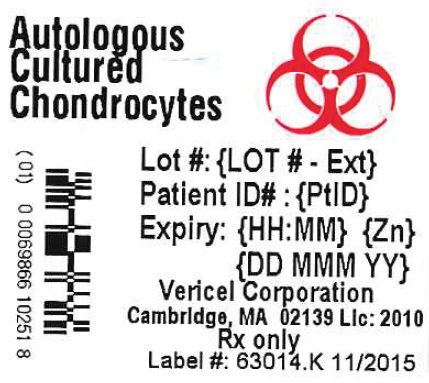
- PRINCIPAL DISPLAY PANEL - 0.4 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 0.4 mL Vial Shipping Label
Autologous Cultured Chondrocytes
Carticel®
FOR AUTOLOGOUS USE ONLY
NOT EVALUATED FOR INFECTIOUS SUBSTANCES
Vericel Corporation Patient ID #: {Patient ID}
{Patient Initials}
{Hospital Name}
Lot #:{LOT # - Ext}
Expiry: {HH:MM} {Zn} {DD MMM YY}
Store at room temperature. DO NOT FREEZE
({#})vial(s) approximately 12 million cells per vial in 0.4 mL DMEM
for implantation. No US Standard of Potency. NO PRESERVATIVES
Cells Expanded in Medium Containing 44 mcg/mL Gentamicin
See Insert for Complete Instructions and Dosing
Rx Only
Vericel Corporation
Cambridge, MA 02139 USA
Carticel® is a registered trademark of Vericel Corporation
Label #: 64014.J - 11/2015
License #: 2010
-
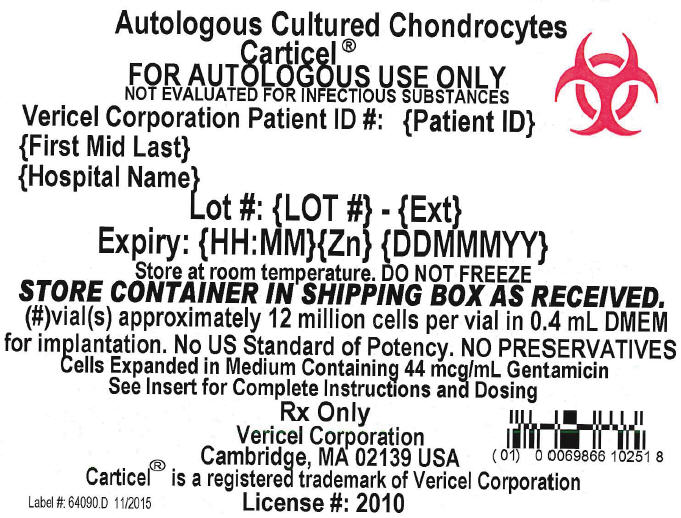
Principal Display Panel - 0.4 mL Vial Carton Label
Autologous Cultured Chondrocytes
Carticel®
FOR AUTOLOGOUS USE ONLY
NOT EVALUATED FOR INFECTIOUS SUBSTANCES
Vericel Corporation Patient ID #: {Patient ID}
{First Mid Last}
{Hospital Name}
Lot #: {LOT #} - {Ext}
Expiry: {HH:MM}{Zn} {DDMMMYY}
Store at room temperature. DO NOT FREEZE
STORE CONTAINER IN SHIPPING BOX AS RECEIVED.
{#}vial(s) approximately 12 million cells per vial in 0.4 mL DMEM
for implantation. No US Standard of Potency. NO PRESERVATIVES
Cells Expanded in Medium Containing 44 mcg/mL Gentamicin
See Insert for Complete Instructions and Dosing
Rx Only
Vericel Corporation
Cambridge, MA 02139 USA
Carticel® is a registered trademark of Vericel Corporation
Label #: 64090.D 11/2015
License #: 2010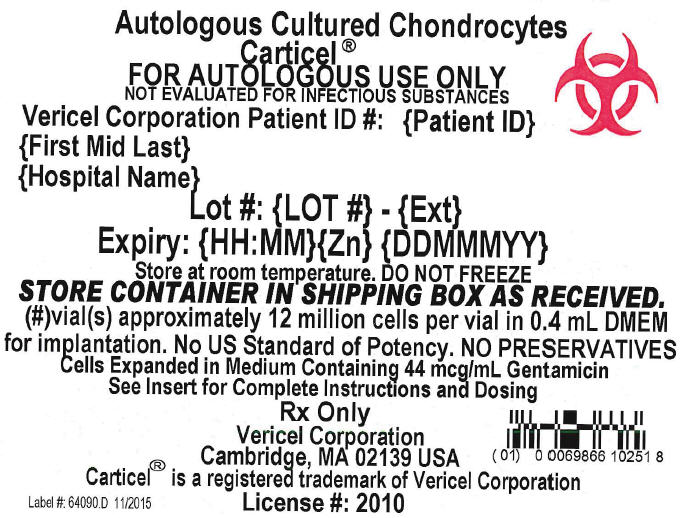
-
INGREDIENTS AND APPEARANCE
CARTICEL
autologous cultured chondrocytes implantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69866-1025 Route of Administration INTRA-ARTICULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength autologous cultured chondrocytes (UNII: D5P3K3V822) (autologous cultured chondrocytes - UNII:D5P3K3V822) autologous cultured chondrocytes 1.2e+007 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69866-1025-1 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product 11/17/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103661 06/19/1905 Labeler - Vericel Corporation (079745570) Establishment Name Address ID/FEI Business Operations Vericel Corporation 079745570 API MANUFACTURE(69866-1025) , MANUFACTURE(69866-1025)