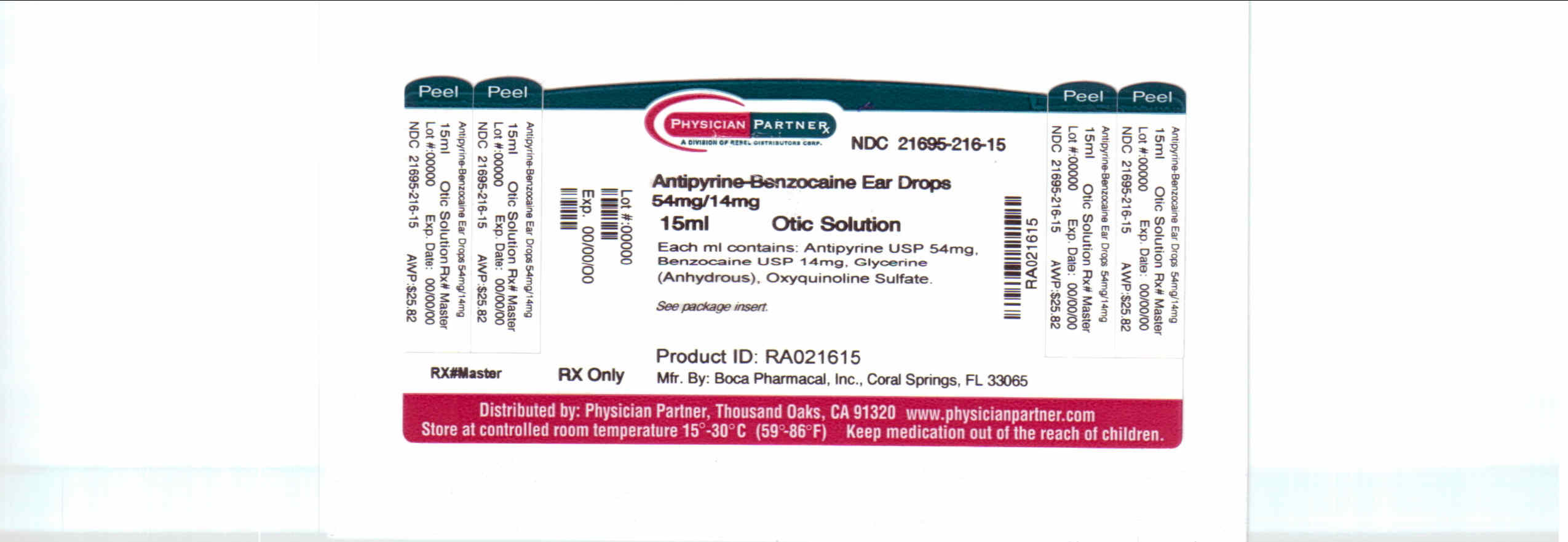
Label: ANTIPYRINE AND BENZOCAINE solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-216-10, 21695-216-15 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 64376-438
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 17, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Antipyrine and Benzocaine Otic Solution is an otic solution containing Antipyrine, Benzocaine, Oxyquinoline Sulfate, and Anhydrous Glycerin for use in the ear. The solution congeals at 0°C (32°F), but returns to normal consistency, unchanged, at room temperature.
Antipyrine is an analgesic with local anesthetic action, it is chemically 2,3-dimethyl-1-phenyl-3-pyrazolin-5- one.
The active ingredient is represented by the structural formula: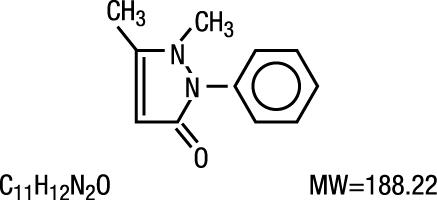
Antipyrine occurs as colorless crystals or white powder, has a slightly bitter taste and is soluble in water and alcohol.
Benzocaine is a local anesthetic. It is chemically ethyl p-aminobenzoate or Benzoic acid, 4-amino-, ethyl ester.
The active ingredient is represented by the structural formula: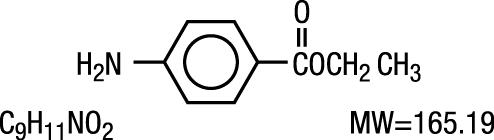
It occurs as white crystals or white crystalline powder and is slightly soluble in water and soluble in organic solvents.
EACH mL CONTAINS:
Actives: Antipyrine 54 mg, Benzocaine 14 mg; Inactives: Glycerine (anhydrous), Oxyquinoline Sulfate.
-
CLINICAL PHARMACOLOGY
Antipyrine and Benzocaine Otic Solution combines the hygroscopic property of anhydrous glycerin with the analgesic action of antipyrine and benzocaine to relieve pressure, reduce inflammation and congestion, and to alleviate pain and discomfort in acute otitis media.
Antipyrine and Benzocaine Otic Solution does not blanch the tympanic membrane or mask the landmarks and, therefore, does not distort the otoscopic picture.
-
INDICATIONS AND USAGE
Acute Otitis media of various etiologies
-prompt relief of pain and reduction of inflammation in the congestive and serous stages.
-adjuvant therapy during systemic antibiotic administration for resolution of the infection.
Because of the close anatomical relationship of the eustachian tube to the nasal cavity, otitis media is a frequent problem, especially in children in whom the tube is shorter, wider, and more horizontal than in adults.
Removal of Cerumen
-facilitates the removal of excessive or impacted cerumen.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Information for Patients: Avoid contaminating the dropper with material from the ear, fingers or other source.
Pregnancy
Pregnancy: Category C. Animal reproduction studies have not been conducted with Antipyrine and Benzocaine Otic Solution.
It is also not known whether Antipyrine and Benzocaine Otic Solution can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Antipyrine and Benzocaine
Otic Solution should be given to a pregnant woman only if clearly needed. -
DOSAGE AND ADMINISTRATION
Acute otitis media: Instill Antipyrine and Benzocaine Otic Solution, permitting the solution to run along the wall of the ear canal until it is filled. Avoid touching the ear with dropper. Then moisten a cotton pledget with Antipyrine and Benzocaine Otic Solution and insert into meatus. Repeat every one to two hours until pain and congestion are relieved.
Removal of Cerumen:
Before: Instill Antipyrine and Benzocaine Otic Solution three times daily for two or three days to help detach cerumen from wall of ear canal and facilitate removal.
After: Antipyrine and Benzocaine Otic Solution is useful for drying out the ear canal or relieving discomfort.
Before and after removal of cerumen, a cotton pledget moistened with Antipyrine and Benzocaine Otic Solution should be inserted into the meatus following instillation.
-
HOW SUPPLIED
Antipyrine and Benzocaine Otic Solution NDC 21695-216-15 is supplied in a 15 mL light-resistant dropper-bottle with a screwtop bottle cap. Tamper evident seal on bottle cap. Do not use if seal is broken.
Storage and Handling
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature]. Protect from heat and light. Protect from freezing.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
Manufactured for:
Boca Pharmacal, Inc.
Coral Springs, FL 33065
www.bocapharmacal.com
1-800-354-8460
Rev. 04/09
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTIPYRINE AND BENZOCAINE
antipyrine and benzocaine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-216(NDC:64376-438) Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIPYRINE (UNII: T3CHA1B51H) (ANTIPYRINE - UNII:T3CHA1B51H) ANTIPYRINE 54 mg in 1 mL BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 14 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) OXYQUINOLINE SULFATE (UNII: 61VUG75Y3P) Product Characteristics Color WHITE (off) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-216-10 10 mL in 1 BOTTLE, DROPPER 2 NDC:21695-216-15 15 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 04/30/2009 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK