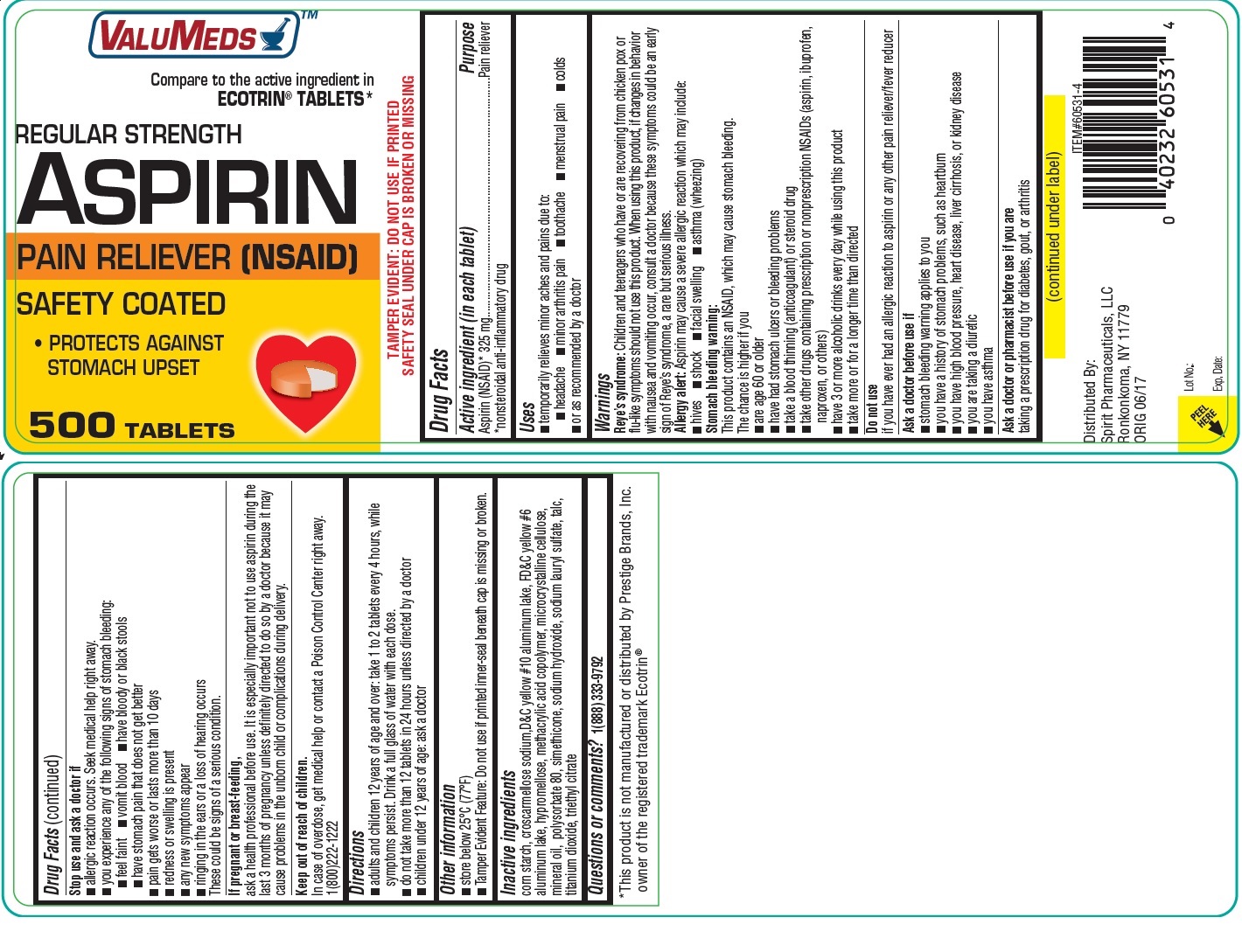
Label: VALUMEDS ASPIRIN- aspirin tablet, delayed release
- NDC Code(s): 68210-0025-0
- Packager: SPIRIT PHARMACEUTICALS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- INDICATIONS & USAGE
-
WARNINGS
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- shock
- facial swelling
- asthma (wheezing)
Stomach bleeding warning: This product contains an NSAID, which may cause stomach bleeding.
The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDS(aspirin, ibuprofen, naproxen or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed.
- Do not use
- Ask a doctor before use if
- Ask a doctor or phamracist before use if you are
-
Stop use and ask a doctor if
- allergic reaction occurs, Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- redness or swelling is present
- any new symptom appear
- ringing in the ears or a loss of hearing occurs
These could be signs of a serious condition.
- If pregnant or breast-feeding,
- Keep out of reach of children
- Directions
- Other information
- INACTIVE INGREDIENT
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VALUMEDS ASPIRIN
aspirin tablet, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68210-0025 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color orange Score no score Shape ROUND Size 10mm Flavor Imprint Code T Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68210-0025-0 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/17/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 04/17/2018 Labeler - SPIRIT PHARMACEUTICALS LLC (179621011)