Label: CINACALCET tablet, film coated
- NDC Code(s): 67877-503-01, 67877-503-30, 67877-503-33, 67877-504-01, view more
- Packager: Ascend Laboratories, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CINACALCET TABLETS safely and effectively. See full prescribing information for CINACALCET TABLETS. CINACALCET tablets , for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGE1.1 Secondary Hyperparathyroidism - Cinacalcet are indicated for the treatment of secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on dialysis [see Clinical ...
-
2 DOSAGE & ADMINISTRATION2.1 Administration - Cinacalcet Tablets should be taken with food or shortly after a meal. Cinacalcet Tablets are administered orally and should always be taken whole and not chewed, crushed ...
-
3 DOSAGE FORMS & STRENGTHSCinacalcet tablets is available as film-coated tablets. Cinacalcet tablets are formulated as light green colored, oval shaped, film coated tablets debossed with “A33”, “A34” , “A35” on one side ...
-
4 CONTRAINDICATIONSCinacalcet treatment initiation is contraindicated if serum calcium is less than the lower limit of the normal range [see Warnings and Precautions (5.1)].
-
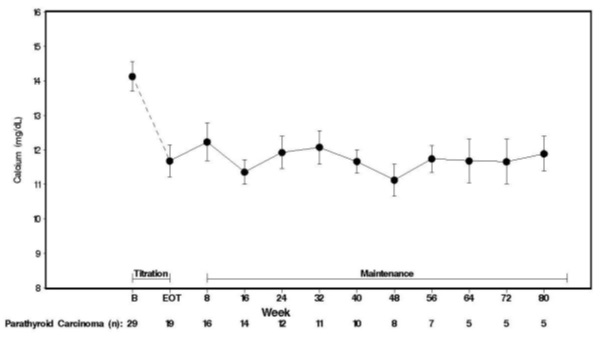
5 WARNINGS AND PRECAUTIONS5.1 Hypocalcemia - Cinacalcet lowers serum calcium and can lead to hypocalcemia [see Adverse Reactions (6.1)] .Significant lowering of serum calcium can cause paresthesias, myalgias, muscle ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of labeling: Hypocalcemia [see Warnings and Precautions (5.1)] Upper Gastrointestinal Bleeding [see Warnings ...
-
7 DRUG INTERACTIONS7.1 Strong CYP3A4 Inhibitors - Cinacalcet is partially metabolized by CYP3A4. Dose adjustment of cinacalcet may be required if a patient initiates or discontinues therapy with a strong CYP3A4 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited case reports of cinacalcet use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. In animal reproduction ...
-
10 OVERDOSAGEOverdosage of cinacalcet may lead to hypocalcemia. In the event of overdosage, patients should be monitored for signs and symptoms of hypocalcemia and appropriate measures taken to correct serum ...
-
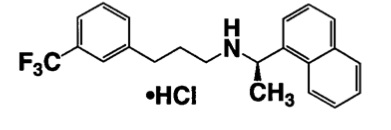
11 DESCRIPTIONCinacalcet tablets contain the hydrochloride salt of the active ingredient cinacalcet, a positive modulator of the calcium sensing receptor. The empirical formula for cinacalcet is C22H22F3N⋅HCl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The calcium-sensing receptor on the surface of the chief cell of the parathyroid gland is the principal regulator of PTH synthesis and secretion. Cinacalcet, the active ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis, Impairment Of Fertility - Carcinogenicity - Standard lifetime dietary carcinogenicity bioassays were conducted in mice and rats. Mice were given cinacalcet at ...
-
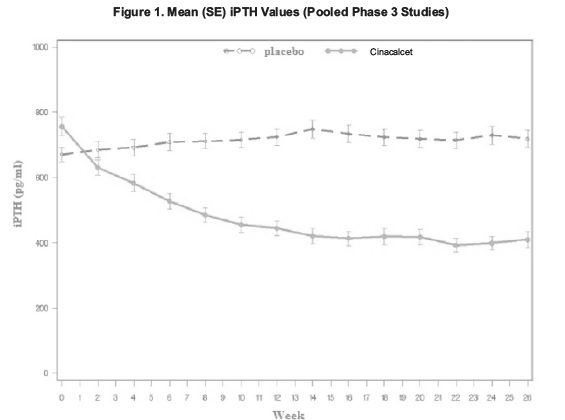
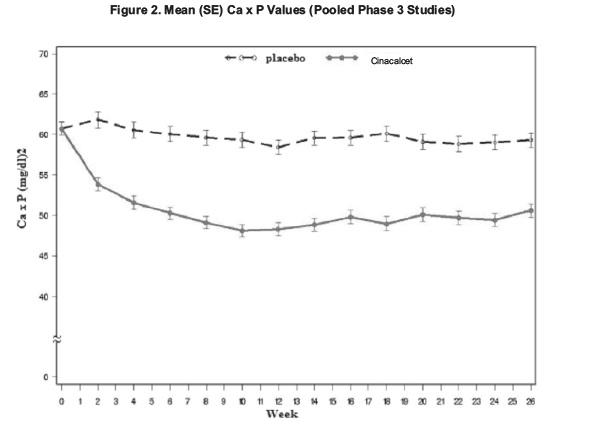
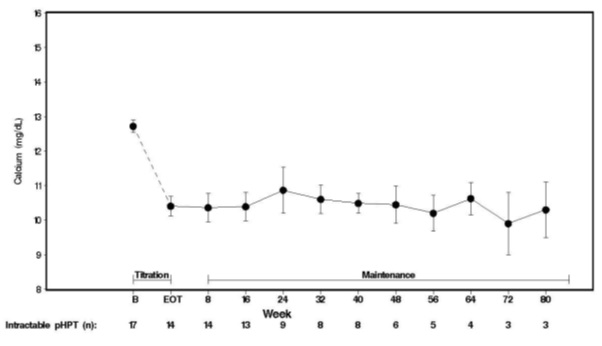
14 CLINICAL STUDIES14.1 Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis - Three 6-month, multicenter, randomized, double-blind, placebo-controlled clinical studies of similar ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCinacalcet Tablets 30 mg are formulated as light green colored, oval shaped, film coated tablets debossed with “A33” on one side and “30” on other side, packaged in- bottles of 30 tablets (NDC ...
-
17 PATIENT COUNSELING INFORMATIONHypocalcemia: Advise patients to report symptoms of hypocalcemia, including paresthesias, myalgias, muscle spasms, and seizures, to their healthcare provider [see Warnings and Precautions ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELAscend Laboratories, LLC - NDC 67877-503-30 - Cinacalcet Tablets - 30 mg - Rx only - 30 tablets - Ascend Laboratories, LLC - NDC 67877-504-30 - Cinacalcet Tablets - 60 mg - Rx only - 30 tablets - Ascend ...
-
INGREDIENTS AND APPEARANCEProduct Information