Label: LEVOTHYROXINE SODIUM injection, solution
- NDC Code(s): 63323-885-10, 63323-890-10, 63323-895-10
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 5, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LEVOTHYROXINE SODIUM INJECTION safely and effectively.
See full prescribing information for LEVOTHYROXINE SODIUM INJECTION.
LEVOTHYROXINE SODIUM injection, for intravenous use
Initial U.S. Approval: 2002INDICATIONS AND USAGE
Levothyroxine Sodium Injection is L-thyroxine (T4) indicated in adult patients for the treatment of myxedema coma. (1)
Limitations of Use:
Not recommended as a substitute for oral levothyroxine sodium because the relative bioavailability of Levothyroxine Sodium Injection to oral levothyroxine sodium has not been established and there is a risk of inaccurate dose conversion. (1)
DOSAGE AND ADMINISTRATION
- Consider the age, general physical condition, cardiac risk factors, and clinical severity of myxedema and duration of myxedema symptoms when determining dosages of Levothyroxine Sodium Injection. (2.1)
- Start with lower doses in elderly patients and in patients with underlying cardiovascular disease. (2.1)
- The recommended loading dose is 300 mcg to 500 mcg administered intravenously. (2.1)
- The recommended maintenance dose is 50 mcg to 100 mcg administered intravenously daily until the patient can tolerate oral therapy. (2.1)
- Administer Levothyroxine Sodium Injection intravenously at a rate not to exceed 100 mcg per minute. (2.2)
- Do not add Levothyroxine Sodium Injection to intravenous fluids. (2.2)
DOSAGE FORMS AND STRENGTHS
Injection:
- 100 mcg per 5 mL (20 mcg per mL) single-dose vial
- 200 mcg per 5 mL (40 mcg per mL) single-dose vial
- 500 mcg per 5 mL (100 mcg per mL) single-dose vial (3)
CONTRAINDICATIONS
Uncorrected adrenal insufficiency. (4)
WARNINGS AND PRECAUTIONS
- Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease: Overtreatment may cause arrhythmias, tachycardia, myocardial ischemia and infarction, or worsening of congestive heart failure and death, particularly in patients with cardiovascular disease and in elderly patients. Start with lower doses in elderly patients and in patients with underlying cardiovascular disease and monitor patients after administration (5.1).
- Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency: Initiation of thyroid hormone therapy prior to initiating glucocorticoid therapy may precipitate an acute adrenal crisis in patients with adrenal insufficiency. Treat patients with adrenal insufficiency with replacement glucocorticoids prior to initiating treatment (5.2).
- Worsening of Diabetic Control: May worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control (5.3).
ADVERSE REACTIONS
Adverse reactions associated with Levothyroxine Sodium Injection are primarily those of hyperthyroidism due to therapeutic overdosage: fatigue, increased appetite, weight loss, heat intolerance, fever, excessive sweating, headache, hyperactivity, nervousness, anxiety, irritability, emotional lability, insomnia, tremors, muscle weakness, muscle spasm, palpitations, tachycardia, arrhythmias, increased pulse and blood pressure, heart failure, angina, myocardial infarction, cardiac arrest, dyspnea, diarrhea, vomiting, abdominal cramps, elevations in liver function tests, flushing, and rash. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See full prescribing information for drugs that affect thyroid hormone pharmacokinetics and metabolism (e.g., synthesis, secretion, catabolism, protein binding, and target tissue response) that may alter the therapeutic response to Levothyroxine Sodium Injection. (7)
Revised: 4/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease
5.2 Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency
5.3 Worsening of Diabetic Control
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics
7.2 Antidiabetic Therapy
7.3 Oral Anticoagulants
7.4 Digitalis Glycosides
7.5 Antidepressant Therapy
7.6 Ketamine
7.7 Sympathomimetics
7.8 Drug-Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- BOXED WARNING (What is this?)
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
- Consider the age, general physical condition, cardiac risk factors, and clinical severity of myxedema and duration of myxedema symptoms when determining the starting and maintenance dosages of Levothyroxine Sodium Injection.
- Start with lower doses in elderly patients and in patients with underlying cardiovascular disease [see Warnings and Precautions (5.1) and Use in Specific Populations (8.5)].
- The recommended loading dose of Levothyroxine Sodium Injection is 300 mcg to 500 mcg administered intravenously.
- The recommended maintenance dose of Levothyroxine Sodium Injection is 50 mcg to 100 mcg administered intravenously daily until the patient can tolerate oral therapy.
2.2 Administration Instructions
- Administer Levothyroxine Sodium Injection as an intravenous injection at a rate not to exceed 100 mcg per minute.
- Do not add Levothyroxine Sodium Injection to intravenous fluids.
- Inspect Levothyroxine Sodium Injection visually prior to injection. It should appear clear and colorless, solution free of visible particulates. Do not use if particulate matter or coloration is seen.
- Discard any unused portion.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease
Overtreatment with Levothyroxine Sodium Injection may cause arrhythmias, tachycardia, myocardial ischemia and infarction, or worsening of congestive heart failure and death, particularly in patients with cardiovascular disease and in elderly patients. Start with lower doses in elderly patients and in patients with underlying cardiovascular disease and monitor patients after administration of Levothyroxine Sodium Injection for cardiac adverse reactions.
5.2 Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency
Chronic autoimmune thyroiditis, which can lead to myxedema coma, may occur in association with other autoimmune disorders such as adrenal insufficiency. Thyroid hormone increases metabolic clearance of glucocorticoids. Initiation of thyroid hormone therapy prior to initiating glucocorticoid therapy may precipitate an acute adrenal crisis in patients with adrenal insufficiency [see Contraindications (4)]. Treat patients with adrenal insufficiency with replacement glucocorticoids prior to initiating treatment with Levothyroxine Sodium Injection.
5.3 Worsening of Diabetic Control
Addition of levothyroxine therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control [see Drug Interactions (7.2)].
-
6 ADVERSE REACTIONS
Adverse reactions associated with levothyroxine are primarily those of hyperthyroidism due to therapeutic overdosage [see Warnings and Precautions (5), Overdosage (10)]. They include the following:
- General: fatigue, increased appetite, weight loss, heat intolerance, fever, excessive sweating
- Central nervous system: headache, hyperactivity, nervousness, anxiety, irritability, emotional lability, insomnia
- Musculoskeletal: tremors, muscle weakness, muscle spasm
- Cardiovascular: palpitations, tachycardia, arrhythmias, increased pulse and blood pressure, heart failure, angina, myocardial infarction, cardiac arrest
- Respiratory: dyspnea
- Gastrointestinal: diarrhea, vomiting, abdominal cramps, elevations in liver function tests
- Dermatologic: flushing, rash
Seizures have been reported rarely with the institution of levothyroxine therapy.
Hypersensitivity Reactions
Hypersensitivity reactions to inactive ingredients have occurred in patients treated with thyroid hormone products. These include urticaria, pruritus, skin rash, flushing, angioedema, various gastrointestinal symptoms (abdominal pain, nausea, vomiting and diarrhea), fever, arthralgia, serum sickness, and wheezing. Hypersensitivity to levothyroxine itself is not known to occur.
-
7 DRUG INTERACTIONS
7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics
Many drugs affect thyroid hormone pharmacokinetics and metabolism (e.g., synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to Levothyroxine Sodium Injection (see Tables 1-3).
Table 1: Drugs That May Alter T4 and Triiodothyronine (T3) Serum Transport Without Effecting Free Thyroxine (FT4) Concentration (Euthyroidism) Drug or Drug Class Effect Clofibrate
Estrogen-containing oral contraceptives
Estrogens (oral)
Heroin / Methadone
5-Fluorouracil
Mitotane
TamoxifenThese drugs may increase serum thyroxine-binding globulin (TBG) concentration. Androgens / Anabolic Steroids
Asparaginase
Glucocorticoids
Slow-Release Nicotinic AcidThese drugs may decrease serum TBG concentration. Potential impact (below): Administration of these agents with levothyroxine results in an initial transient increase in FT4. Continued administration results in a decrease in serum T4 and normal FT4 and TSH concentrations. Salicylates (> 2 g/day)
Salicylates inhibit binding of T4 and T3 to TBG and transthyretin. An initial increase in serum FT4 is followed by return of FT4 to normal levels with sustained therapeutic serum salicylate concentrations, although total T4 levels may decrease by as much as 30%. Other drugs:
Carbamazepine
Furosemide (> 80 mg IV)
Heparin
Hydantoins
Non-Steroidal Anti-inflammatory Drugs
- FenamatesThese drugs may cause protein-binding site displacement. Furosemide has been shown to inhibit the protein binding of T4 to TBG and albumin, causing an increase free T4 fraction in serum. Furosemide competes for T4-binding sites on TBG, prealbumin, and albumin, so that a single high dose can acutely lower the total T4 level. Phenytoin and carbamazepine reduce serum protein binding of levothyroxine, and total and free T4 may be reduced by 20% to 40%, but most patients have normal serum TSH levels and are clinically euthyroid. Closely monitor thyroid hormone parameters. Table 2: Drugs That May Alter Hepatic Metabolism of T4 (Hypothyroidism) Potential impact: Stimulation of hepatic microsomal drug-metabolizing enzyme activity may cause increased hepatic degradation of levothyroxine, resulting in increased levothyroxine requirements. Drug or Drug Class Effect Phenobarbital
RifampinPhenobarbital has been shown to reduce the response to thyroxine. Phenobarbital increases L-thyroxine metabolism by inducing uridine 5'-diphospho-glucuronosyltransferase (UGT) and leads to a lower T4 serum levels. Changes in thyroid status may occur if barbiturates are added or withdrawn from patients being treated for hypothyroidism. Rifampin has been shown to accelerate the metabolism of levothyroxine. Table 3: Drugs That May Decrease Conversion of T4 to T3 Potential impact: Administration of these enzyme inhibitors decreases the peripheral conversion of T4 to T3, leading to decreased T3 levels. However, serum T4 levels are usually normal but may occasionally be slightly increased. Drug or Drug Class Effect Beta-adrenergic antagonists
(e.g., Propranolol > 160 mg/day)
In patients treated with large doses of propranolol (> 160 mg/day), T3 and T4 levels change slightly, TSH levels remain normal, and patients are clinically euthyroid. It should be noted that actions of particular beta-adrenergic antagonists may be impaired when the hypothyroid patient is converted to the euthyroid state. Glucocorticoids
(e.g., Dexamethasone ≥ 4 mg/day)
Short-term administration of large doses of glucocorticoids may decrease serum T3 concentrations by 30% with minimal change in serum T4 levels. However, long-term glucocorticoid therapy may result in slightly decreased T3 and T4 levels due to decreased TBG production (See above). Other drugs:
AmiodaroneAmiodarone inhibits peripheral conversion of levothyroxine (T4) to triiodothyronine (T3) and may cause isolated biochemical changes (increase in serum free-T4, and decreased or normal free-T3) in clinically euthyroid patients. 7.2 Antidiabetic Therapy
Addition of levothyroxine to antidiabetic or insulin therapy may result in increased antidiabetic agent or insulin requirements. Careful monitoring of glycemic control is recommended.
7.3 Oral Anticoagulants
Levothyroxine increases the response to oral anticoagulant therapy. Therefore, a decrease in the dose of anticoagulant may be warranted with correction of the hypothyroid state. Closely monitor coagulation tests to permit appropriate and timely dosage adjustments.
7.4 Digitalis Glycosides
Levothyroxine may reduce the therapeutic effects of digitalis glycosides. Serum digitalis glycoside levels may be decreased when a hypothyroid patient becomes euthyroid, necessitating an increase in the dose of digitalis glycosides.
7.5 Antidepressant Therapy
Concurrent use of tricyclic (e.g., amitriptyline) or tetracyclic (e.g., maprotiline) antidepressants and levothyroxine may increase the therapeutic and toxic effects of both drugs, possibly due to increased receptor sensitivity to catecholamines. Toxic effects may include increased risk of cardiac arrhythmias and central nervous system stimulation. Levothyroxine may accelerate the onset of action of tricyclics. Administration of sertraline in patients stabilized on levothyroxine may result in increased levothyroxine requirements.
7.6 Ketamine
Concurrent use of ketamine and levothyroxine may produce marked hypertension and tachycardia. Closely monitor blood pressure and heart rate in these patients.
7.7 Sympathomimetics
Concurrent use may of sympathomimetics and levothyroxine may increase the effects of sympathomimetics or thyroid hormone. Thyroid hormones may increase the risk of coronary insufficiency when sympathomimetic agents are administered to patients with coronary artery disease.
7.8 Drug-Laboratory Test Interactions
Consider changes in TBG concentration when interpreting T4 and T3 values. Measure and evaluate unbound (free) hormone and/or determine the free T4 index (FT4I) in this circumstance. Pregnancy, infectious hepatitis, estrogens, estrogen containing oral contraceptives, and acute intermittent porphyria increase TBG concentrations. Nephrosis, severe hypoproteinemia, severe liver disease, acromegaly, androgens, and corticosteroids decrease TBG concentration. Familial hyper- or hypo-thyroxine binding globulinemias have been described, with the incidence of TBG deficiency approximating 1 in 9,000.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There is no available data with use of Levothyroxine Sodium Injection in pregnant women. The clinical data in pregnant women treated with oral levothyroxine to maintain a euthyroid state have not reported increased rates of major birth defects, miscarriages, or adverse maternal or fetal outcomes (see Data). There are risks to the mother and fetus associated with myxedema coma in pregnancy (see Clinical Considerations). Animal reproduction studies have not been conducted with levothyroxine sodium.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Myxedema coma is a medical emergency which can be fatal, if left untreated. Delaying treatment in pregnant women with myxedema coma increases the risk of maternal and fetal morbidity and mortality. Life-sustaining therapy for the pregnant woman should not be withheld due to potential concerns regarding the effects of Levothyroxine Sodium Injection on the fetus.
Data
Human Data
There is no available data with use of Levothyroxine Sodium Injection in pregnant women. Oral levothyroxine is approved for use as a replacement therapy in hypothyroidism. There is a long experience of oral levothyroxine use in pregnant women that has not reported increased rates of fetal malformations, miscarriages or other adverse maternal or fetal outcomes associated with levothyroxine use in pregnant women.
8.2 Lactation
Risk Summary
Published studies report that levothyroxine is present in human milk following the administration of oral levothyroxine. However, there is insufficient information to determine the effects of levothyroxine on the breastfed infant and no available information on the effects of levothyroxine on milk production. There is no available data with use of Levothyroxine Sodium Injection in lactating women. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for levothyroxine and any potential adverse effects on the breastfed infant from levothyroxine or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of Levothyroxine Sodium Injection have not been established in pediatric patients.
8.5 Geriatric Use
Because of the increased prevalence of cardiovascular disease among the elderly, initiate Levothyroxine Sodium Injection with lower doses in elderly patients and in patients with underlying cardiovascular disease and closely monitor for cardiac adverse reactions. Atrial arrhythmias can occur in elderly patients. Atrial fibrillation is the most common of the arrhythmias observed with levothyroxine overtreatment in the elderly [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
-
10 OVERDOSAGE
The signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5) and Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral embolism, shock, coma, and death have been reported. Reduce the Levothyroxine Sodium Injection dose or temporarily discontinue if signs or symptoms of overdosage occur. Initiate appropriate supportive treatment as dictated by the patient's medical status.
-
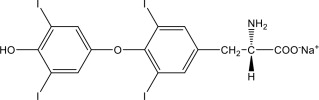
11 DESCRIPTION
Levothyroxine Sodium Injection contains synthetic crystalline levothyroxine (T4) in sodium salt form. Levothyroxine sodium has an empirical formula of C15H10I4NNaO4, a molecular weight of 798.85 g/mol (anhydrous), and the following structural formula:
Levothyroxine Sodium Injection is a sterile, preservative free, clear, colorless, sterile solution for intravenous administration available as: 100 mcg per 5 mL (20 mcg per mL), 200 mcg per 5 mL (40 mcg per mL), and 500 mcg per 5 mL (100 mcg per mL). Each mL of Levothyroxine Sodium Injection also contains 10 mg Tromethamine, USP; 0.14 mg Sodium Iodide, USP; 6.48 mg Sodium Chloride, USP; and Water for Injection, USP Sodium hydroxide, NF and/or Hydrochloric acid, USP may have been added for pH adjustment (9.5 – 10.8). Levothyroxine Sodium Injection is in single dose clear glass vials.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and levothyroxine (T4) diffuse into the cell nucleus and bind to thyroid receptor proteins attached to DNA. This hormone nuclear receptor complex activates gene transcription and synthesis of messenger RNA and cytoplasmic proteins.
The physiological actions of thyroid hormones are produced predominantly by T3, the majority of which (approximately 80%) is derived from T4 by deiodination in peripheral tissues.
12.2 Pharmacodynamics
Levothyroxine sodium is a synthetic T4 hormone that exerts the same physiologic effect as endogenous T4, thereby maintaining normal T4 levels when a deficiency is present.
12.3 Pharmacokinetics
Distribution
Circulating thyroid hormones are greater than 99% bound to plasma proteins, including thyroxine binding globulin (TBG), thyroxine binding prealbumin (TBPA), and albumin (TBA), whose capacities and affinities vary for each hormone. The higher affinity of both TBG and TBPA for T4 partially explains the higher serum levels, slower metabolic clearance, and longer half life of T4 compared to T3. Protein bound thyroid hormones exist in reverse equilibrium with small amounts of free hormone. Only unbound hormone is metabolically active. Many drugs and physiologic conditions affect the binding of thyroid hormones to serum proteins [see Drug Interactions (7)]. Thyroid hormones do not readily cross the placental barrier [see Use in Specific Populations (8.1)].
Metabolism
T4 is slowly eliminated. The major pathway of thyroid hormone metabolism is through sequential deiodination. Approximately eighty percent of circulating T3 is derived from peripheral T4 by monodeiodination. The liver is the major site of degradation for both T4 and T3, with T4 deiodination also occurring at a number of additional sites, including the kidney and other tissues. Approximately 80% of the daily dose of T4 is deiodinated to yield equal amounts of T3 and reverse T3 (rT3). T3 and rT3 are further deiodinated to diiodothyronine. Thyroid hormones are also metabolized via conjugation with glucuronides and sulfates and excreted directly into the bile and gut where they undergo enterohepatic recirculation.
Excretion
Thyroid hormones are primarily eliminated by the kidneys. A portion of the conjugated hormone reaches the colon unchanged, where it is hydrolyzed and eliminated in feces as the free hormones. Urinary excretion of T4 decreases with age.
Table 1: Pharmacokinetic Parameters of Thyroid Hormones in Euthyroid Patients T4: Levothyroxine
T3: Liothyronine
1 3 to 4 days in hyperthyroidism, 9 to 10 days in hypothyroidism.
2 Includes TBG, TBPA, and TBA.
Hormone Ratio in Thyroglobulin Biologic Potency Half-Life (Days) Protein Binding
(%)2T4 10 to 20 1 6 to 81 99.96 T3 1 4 ≤ 2 99.5 - 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Levothyroxine Sodium Injection is a clear, colorless solution available as follows:
NDC
Total Strength per Total Volume Concentration 63323-885-10 100 mcg per 5 mL 20 mcg per mL 63323-890-10 200 mcg per 5 mL 40 mcg per mL 63323-895-10 500 mcg per 5 mL 100 mcg per mL Protect from light in the original vial in a carton and store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. The unopened vial may be stored for up to 24 hours exposed to indoor lighting outside of the carton. The drug product is preservative free. Discard any unused portion.
Lake Zurich, IL 60047
www.fresenius-kabi.com/us
451617
Issued: April 2019
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LEVOTHYROXINE SODIUM
levothyroxine sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63323-885 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) Levothyroxine Sodium Anhydrous 20 ug in 1 mL Inactive Ingredients Ingredient Name Strength Tromethamine (UNII: 023C2WHX2V) Sodium Iodide (UNII: F5WR8N145C) Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63323-885-10 1 in 1 CARTON 05/29/2018 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210632 05/29/2018 LEVOTHYROXINE SODIUM
levothyroxine sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63323-890 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) Levothyroxine Sodium Anhydrous 40 ug in 1 mL Inactive Ingredients Ingredient Name Strength Tromethamine (UNII: 023C2WHX2V) Sodium Iodide (UNII: F5WR8N145C) Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63323-890-10 1 in 1 CARTON 05/29/2018 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210632 05/29/2018 LEVOTHYROXINE SODIUM
levothyroxine sodium injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63323-895 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) Levothyroxine Sodium Anhydrous 100 ug in 1 mL Inactive Ingredients Ingredient Name Strength Tromethamine (UNII: 023C2WHX2V) Sodium Iodide (UNII: F5WR8N145C) Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63323-895-10 1 in 1 CARTON 05/29/2018 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210632 05/29/2018 Labeler - Fresenius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 023648251 MANUFACTURE(63323-885, 63323-890, 63323-895)