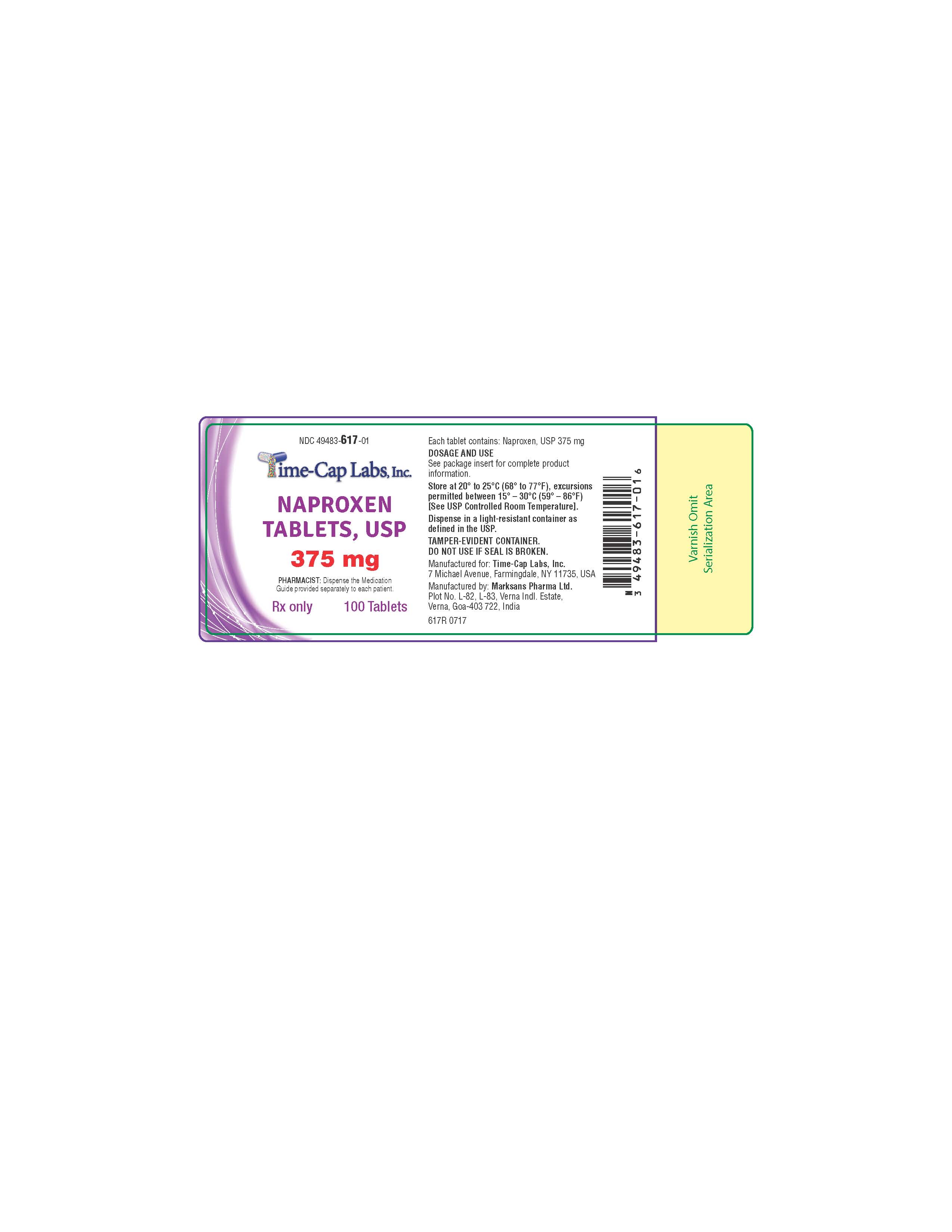
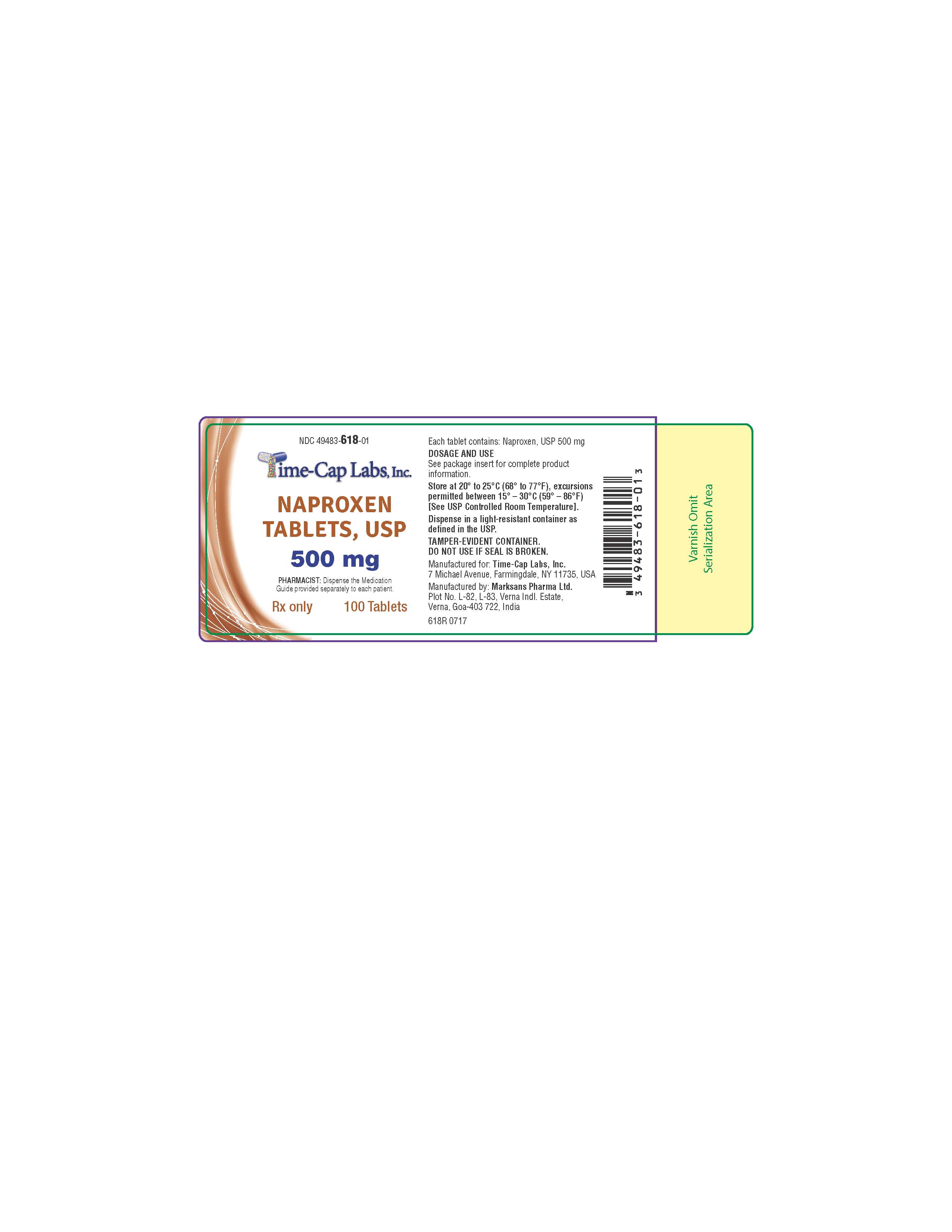
Label: NAPROXEN tablet
-
NDC Code(s):
49483-617-01,
49483-617-50,
49483-618-01,
49483-618-50, view more49483-619-01, 49483-619-50
- Packager: TIME CAP LABORATORIES
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 9, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
INDICATIONS & USAGE
CAREFULLY CONSIDER THE POTENTIAL BENEFITS AND RISKS OF NAPROXEN AND OTHER TREATMENT OPTIONS BEFORE DECIDING TO USE NAPROXEN TABLETS. USE THE LOWEST EFFECTIVE DOSE FOR THE SHORTEST DURATION CONSISTENT WITH INDIVIDUAL PATIENT TREATMENT GOALS (SEE WARNINGS: GASTROINTESTINAL BLEEDING, ULCERATION, AND PERFORATION).
NAPROXEN TABLETS ARE INDICATED:
FOR THE RELIEF OF THE SIGNS AND SYMPTOMS OF RHEUMATOID ARTHRITIS.
FOR THE RELIEF OF THE SIGNS AND SYMPTOMS OF OSTEOARTHRITIS
FOR THE RELIEF OF THE SIGNS AND SYMPTOMS OF ANKYLOSING SPONDYLITIS
FOR THE RELIEF OF THE SIGNS AND SYMPTOMS OF JUVENILE ARTHRITIS
NAPROXEN TABLETS ARE ALSO INDICATED:
FOR RELIEF OF THE SIGNS AND SYMPTOMS OF TENDONITIS
FOR RELIEF OF THE SIGNS AND SYMPTOMS OF BURSITIS
FOR RELIEF OF THE SIGNS AND SYMPTOMS OF ACUTE GOUT
FOR THE MANAGEMENT OF PAIN
FOR THE MANAGEMENT OF PRIMARY DYSMENORRHEA
-
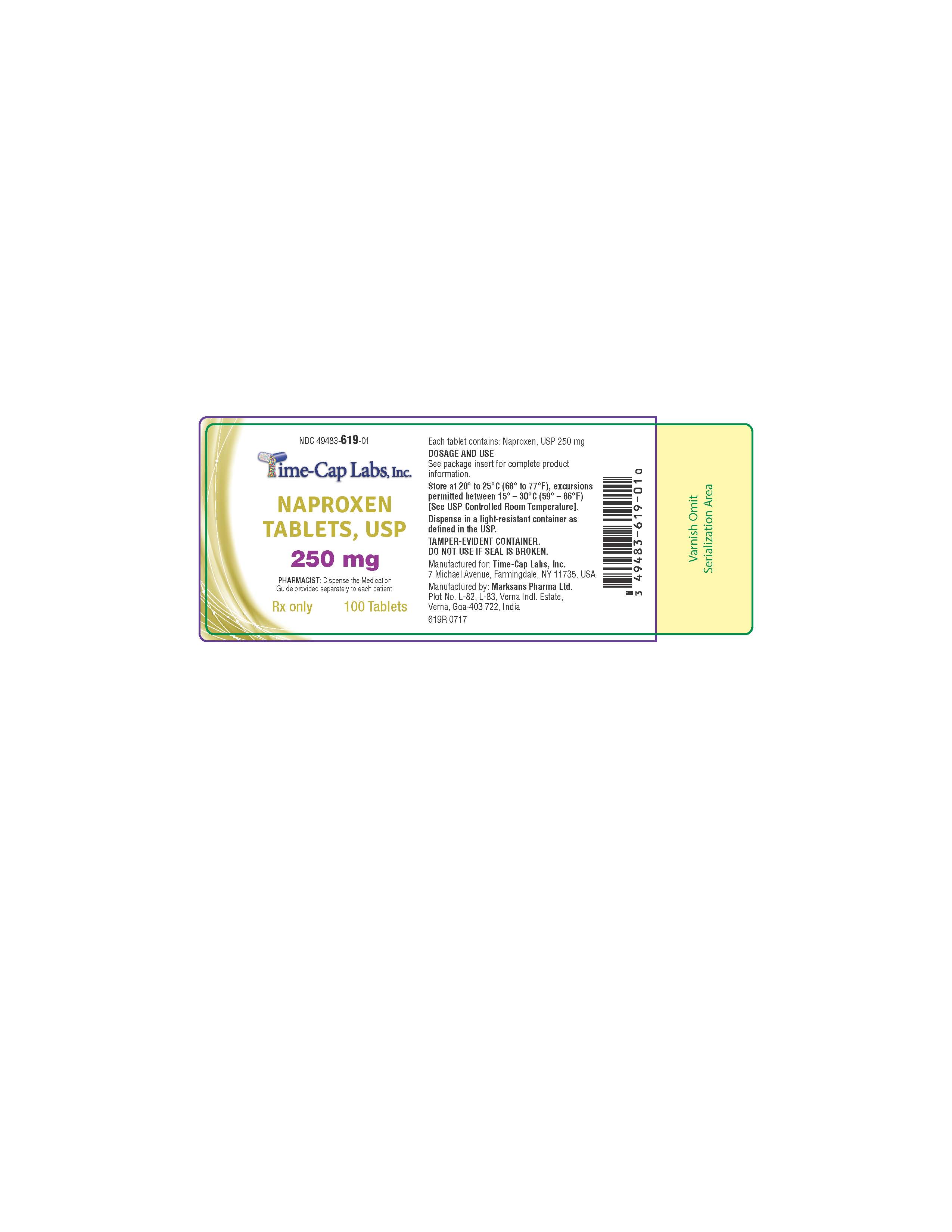
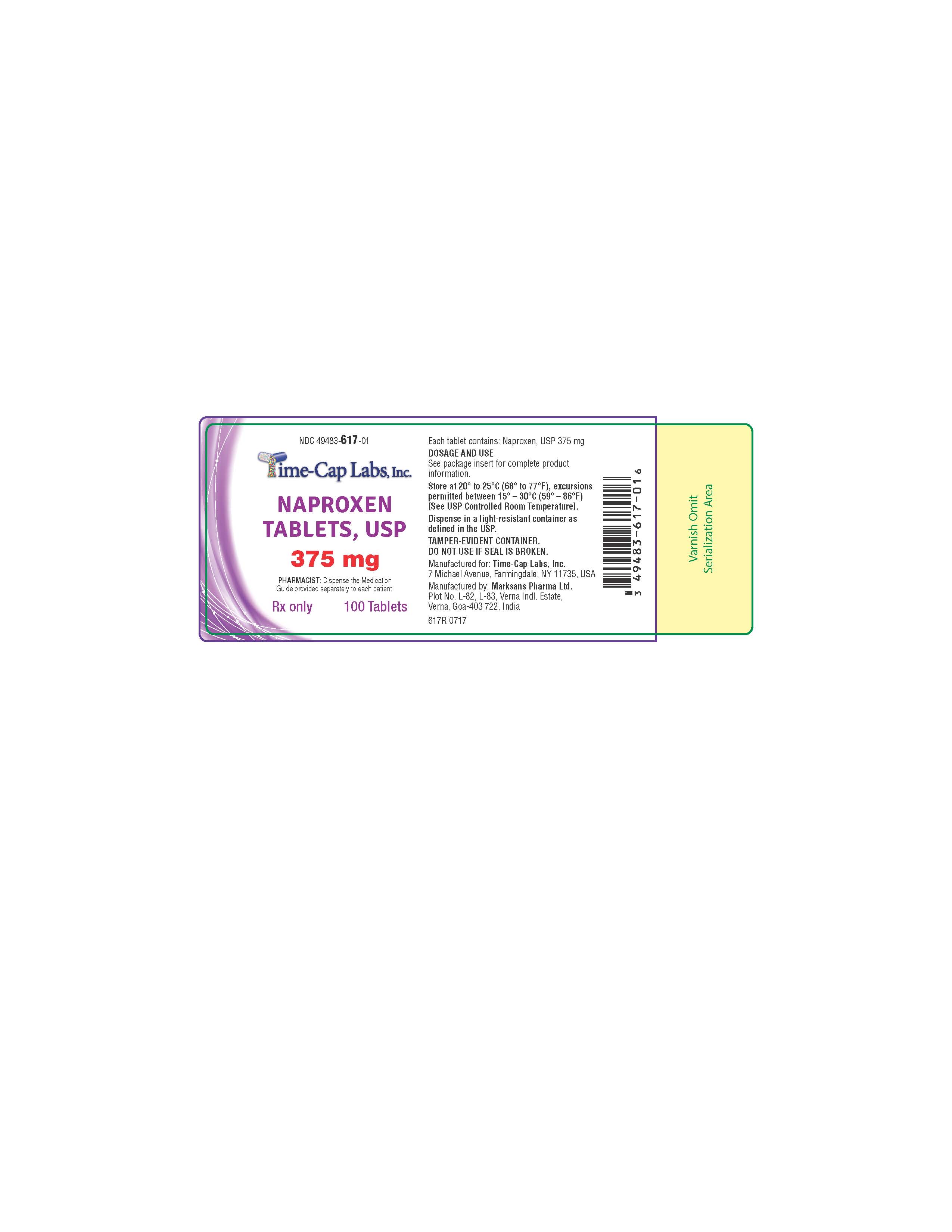
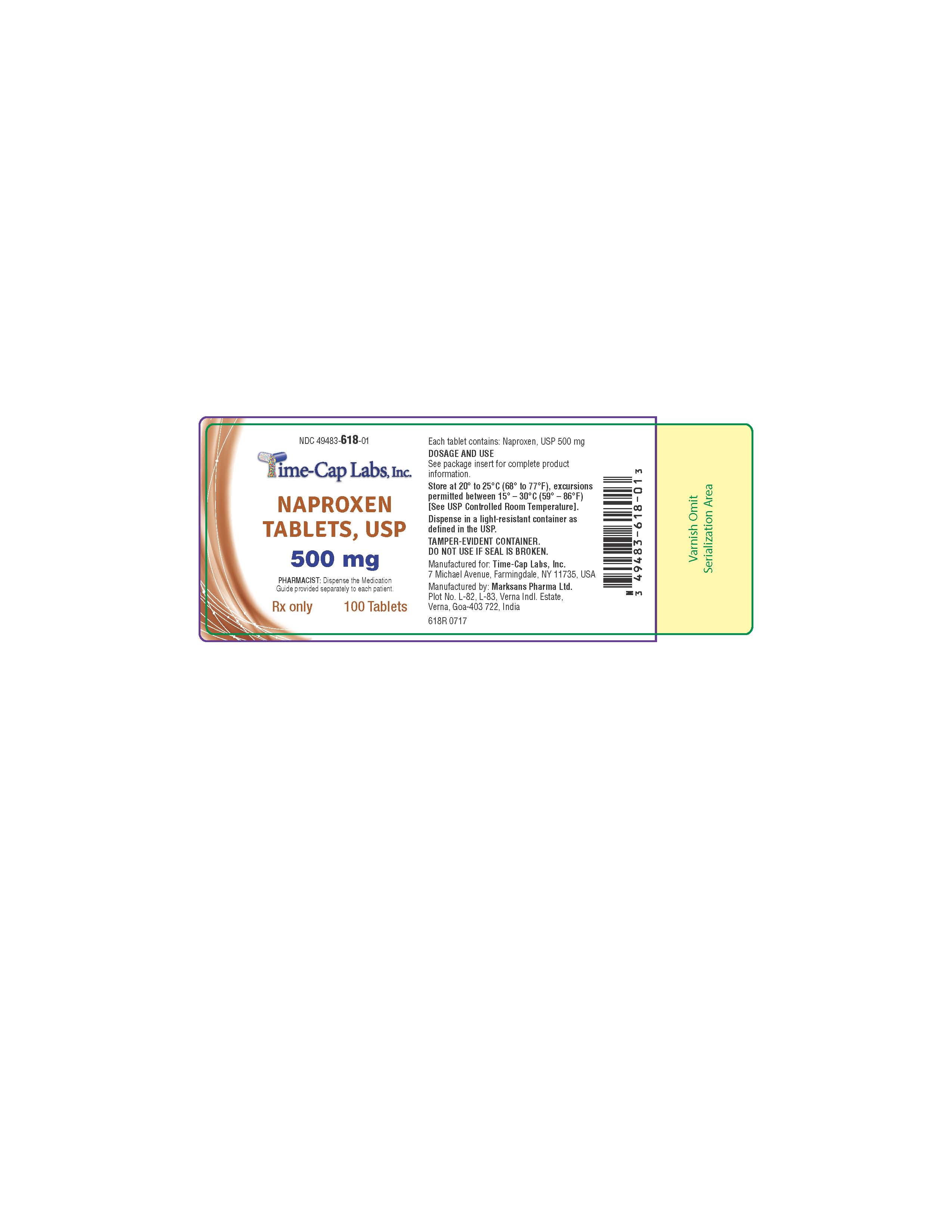
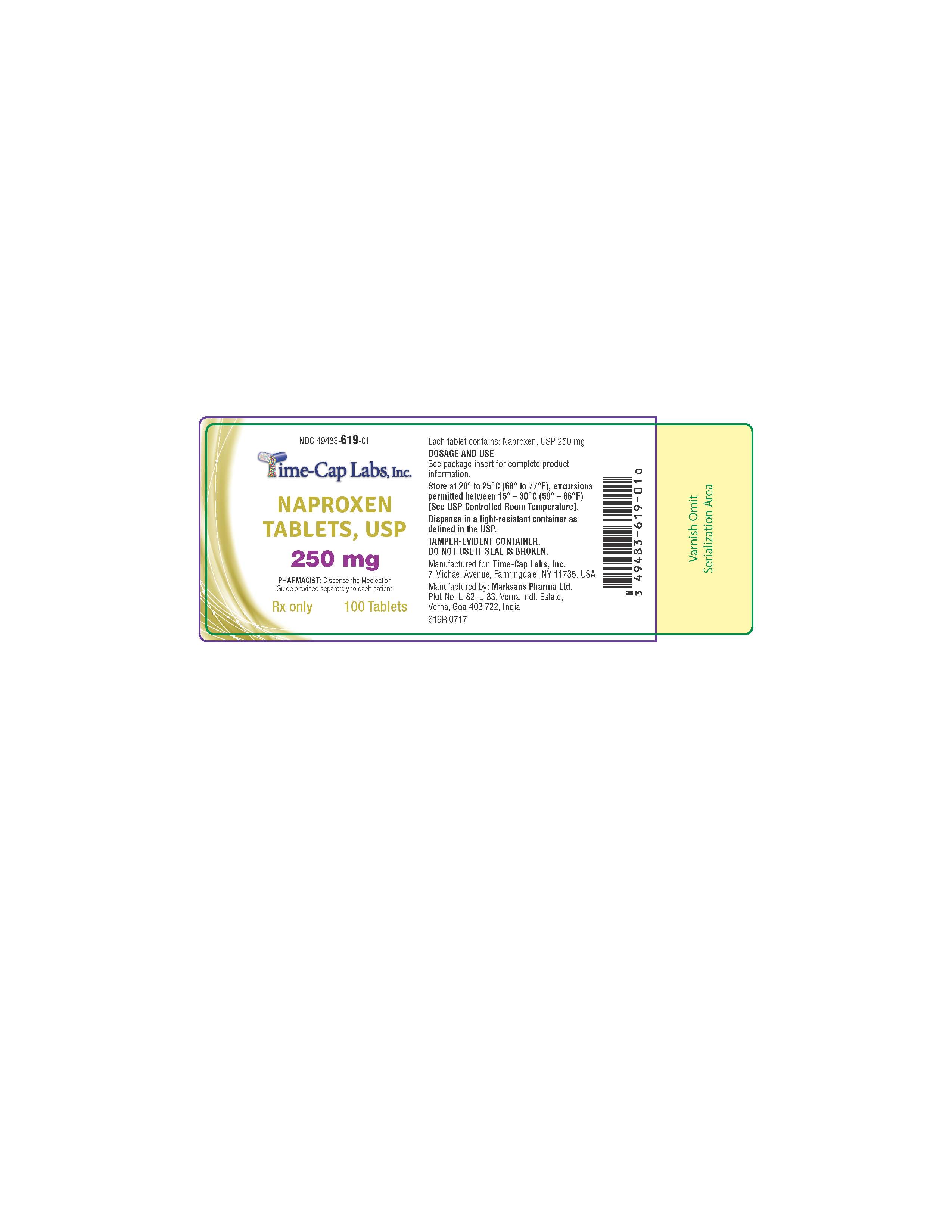
HOW SUPPLIED
NAPROXEN TABLETS
250MG: WHITE TO OFF-WHITE, ROUND SHAPED TABLET WITH “138” DEBOSSED ON ONE SIDE AND SCORED ON OTHER
SIDE. PACKAGED IN LIGHT-RESISTANT BOTTLES OF 100 AND 500.
100'S (BOTTLE): NDC 49483-619-01
500’S (BOTTLE): NDC 49483-619-50
375MG: WHITE TO OFF-WHITE, OVAL SHAPED TABLET WITH “139” DEBOSSED ON ONE SIDE AND PLAIN ON OTHER SIDE.
PACKAGED IN LIGHT-RESISTANT BOTTLES OF 100 AND 1000.
100'S (BOTTLE): NDC 49483-617-01
500’S (BOTTLE): NDC 49483-617-50
500 MG: WHITE TO OFF-WHITE, CAPSULE-SHAPED TABLETS WITH “140” DEBOSSED ON ONE SIDE AND SCORED ON
OTHER SIDE. PACKAGED IN LIGHT-RESISTANT BOTTLES OF 100 AND 500.
100'S (BOTTLE): NDC 49483-618-01
500’S (BOTTLE): NDC 49483-618-50
STORE AT 20°-25°C (68°-77°F) EXCURSIONS PERMITTED TO 15°-30°C (59°-86°F) IN WELL-CLOSED CONTAINERS
[SEE USP CONTROLLED ROOM TEMPERATURE]. DISPENSE IN LIGHT-RESISTANT CONTAINERS.
RX ONLY
-
WARNINGS
CARDIOVAS CULAR THROMBOTIC EVENTS
CLINICAL TRIALS OF SEVERAL COX-2 SELECTIVE AND NON-SELECTIVE NSAIDS OF UP TO THREE YEARS DURATION HAVE SHOWN AN INCREASED RISK OF SERIOUS CARDIOVASCULAR (CV) THROMBOTIC EVENTS, MYOCARDIAL INFARCTION, AND STROKE, WHICH CAN BE FATAL. BASED ON AVAILABLE DATA, IT IS UNCLEAR THAT THE RISK FOR CV THROMBOTIC EVENTS IS SIMILAR FOR ALL NSAIDS. THE RELATIVE INCREASE IN SERIOUS CV THROMBOTIC EVENTS OVER BASELINE CONFERRED BY NSAID USE APPEARS TO BE SIMILAR IN THOSE WITH AND WITHOUT KNOWN CV DISEASE OR RISK FACTORS FOR CV DISEASE. HOWEVER, PATIENTS WITH KNOWN CV DISEASE OR RISK FACTORS HAD A HIGHER ABSOLUTE INCIDENCE OF EXCESS SERIOUS CV THROMBOTIC EVENTS, DUE TO THEIR INCREASED BASELINE RATE. SOMEOBSERVATIONAL STUDIES FOUND THAT THIS INCREASED RISK OF SERIOUS CV THROMBOTIC EVENTS BEGAN AS EARLY AS THE FIRST WEEKS OF TREATMENT. THE INCREASE IN CV THROMBOTIC RISK HAS BEEN OBSERVED MOST CONSISTENTLY AT HIGHER DOSES.
TO MINIMIZE THE POTENTIAL RISK FOR AN ADVERSE CV EVENT IN NSAID-TREATED PATIENTS, USE THE LOWEST EFFECTIVE DOSE FOR THE SHORTEST DURATION POSSIBLE. PHYSICIANS AND PATIENTS SHOULD REMAIN ALERT FOR THE DEVELOPMENT OF SUCH EVENTS, THROUGHOUT THE ENTIRE TREATMENT COURSE, EVEN IN THE ABSENCE OF PREVIOUS CV SYMPTOMS. PATIENTS SHOULD BE INFORMED ABOUT THE SYMPTOMS OF SERIOUS CV EVENTS AND THE STEPS TO TAKE IF THEY OCCUR.
THERE IS NO CONSISTENT EVIDENCE THAT CONCURRENT USE OF ASPIRIN MITIGATES THE INCREASED RISK OF SERIOUS CV THROMBOTIC EVENTS ASSOCIATED WITH NSAID USE. THE CONCURRENT USE OF ASPIRIN AND AN NSAID, SUCH AS NAPROXEN, INCREASES THE RISK OF SERIOUS GASTROINTESTINAL (GI) EVENTS (SEE WARNINGS; GASTROINTESTINAL BLEEDING, ULCERATION, AND PERFORATION).
STATUS POST CORONARY ARTERY BYPASS GRAFT (CABG) SURGERY
TWO LARGE, CONTROLLED, CLINICAL TRIALS OF A COX-2 SELECTIVE NSAID FOR THE TREATMENT OF PAIN IN THE FIRST 10-14 DAYS FOLLOWING CABG SURGERY FOUND AN INCREASED INCIDENCE OF MYOCARDIAL INFARCTION AND STROKE. NSAIDS ARE CONTRAINDICATED IN THE SETTING OF CABG (SEE CONTRAINDICATIONS).
POST-MI PATIENTS
OBSERVATIONAL STUDIES CONDUCTED IN THE DANISH NATIONAL REGISTRY HAVE DEMONSTRATED THAT PATIENTS TREATED WITH NSAIDS IN THE POST-MI PERIOD WERE AT INCREASED RISK OF REINFARCTION, CV-RELATED DEATH, AND ALL CAUSE MORTALITY BEGINNING IN THE FIRST WEEK OF TREATMENT. IN THIS SAME COHORT, THE INCIDENCE OF DEATH IN THE FIRST YEAR POST-MI WAS 20 PER 100 PERSON YEARS IN NSAID-TREATED PATIENTS COMPARED TO 12 PER 100 PERSON YEARS IN NON-NSAID EXPOSED PATIENTS. ALTHOUGH THE ABSOLUTE RATE OF DEATH DECLINED SOMEWHAT
AFTER THE FIRST YEAR POST-MI, THE INCREASED RELATIVE RISK OF DEATH IN NSAID USERS PERSISTED OVER AT LEAST THE NEXT FOUR YEARS OF FOLLOW-UP.
AVOID THE USE OF NAPROXEN IN PATIENTS WITH A RECENT MI UNLESS THE BENEFITS ARE EXPECTED TO OUTWEIGH THE RISK OF RECURRENT CV THROMBOTIC EVENTS. IF NAPROXEN IS USED IN PATIENTS WITH A RECENT MI, MONITOR PATIENTS FOR SIGNS OF CARDIAC ISCHEMIA.
GASTROINTESTINAL BLEEDING, ULCERATION, AND PERFORATION
NSAIDS, INCLUDING NAPROXEN CAUSE SERIOUS GASTROINTESTINAL (GI) ADVERSE EVENTS INCLUDING INFLAMMATION, BLEEDING, ULCERATION, AND PERFORATION OF THE ESOPHAGUS, STOMACH, SMALL INTESTINE, OR LARGE INTESTINE, WHICH CAN BE FATAL. THESE SERIOUS ADVERSE EVENTS CAN OCCUR AT ANY TIME, WITH OR WITHOUT WARNING SYMPTOMS, IN PATIENTS TREATED WITH NSAIDS. ONLY ONE IN FIVE PATIENTS WHO DEVELOP A SERIOUS UPPER GI ADVERSE EVENT ON NSAID THERAPY IS SYMPTOMATIC. UPPER GI ULCERS, GROSS BLEEDING, OR PERFORATION CAUSED BY NSAIDS OCCURRED IN APPROXIMATELY 1% OF PATIENTS TREATED FOR 3-6 MONTHS, AND IN ABOUT 2%-
4% OF PATIENTS TREATED FOR ONE YEAR. HOWEVER, EVEN SHORT-TERM NSAID THERAPY IS NOT WITHOUT RISK. RISK FACTORS FOR GI BLEEDING, ULCERATION, AND PERFORATION PATIENTS WITH A PRIOR HISTORY OF PEPTIC ULCER DISEASE AND/OR GI BLEEDING WHO USED NSAIDS HAD A GREATER
THAN 10-FOLD INCREASED RISK FOR DEVELOPING A GI BLEED COMPARED TO PATIENTS WITHOUT THESE RISK FACTORS. OTHER FACTORS THAT INCREASE THE RISK OF GI BLEEDING IN PATIENTS TREATED WITH NSAIDS INCLUDE LONGER DURATION OF NSAID THERAPY; CONCOMITANT USE OF ORAL CORTICOSTEROIDS, ASPIRIN, ANTICOAGULANTS, OR SELECTIVE SEROTONIN REUPTAKE INHIBITORS (SSRIS); SMOKING; USE OF ALCOHOL; OLDER AGE; AND POOR GENERAL HEALTH STATUS. MOST POSTMARKETING REPORTS OF FATAL GI EVENTS OCCURRED IN ELDERLY OR DEBILITATED PATIENTS.
ADDITIONALLY, PATIENTS WITH ADVANCED LIVER DISEASE AND/OR COAGULOPATHY ARE AT INCREASED RISK FOR GI BLEEDING.
STRATEGIES TO MINIMIZE THE GI RISKS IN NSAID-TREATED PATIENTS:
- USE THE LOWEST EFFECTIVE DOSAGE FOR THE SHORTEST POSSIBLE DURATION.
- AVOID ADMINISTRATION OF MORE THAN ONE NSAID AT A TIME.
- AVOID USE IN PATIENTS AT HIGHER RISK UNLESS BENEFITS ARE EXPECTED TO OUTWEIGH THE INCREASED RISK OF
- BLEEDING. FOR SUCH PATIENTS, AS WELL AS THOSE WITH ACTIVE GI BLEEDING, CONSIDER ALTERNATE THERAPIES OTHER THAN NSAIDS.
- REMAIN ALERT FOR SIGNS AND SYMPTOMS OF GI ULCERATION AND BLEEDING DURING NSAID THERAPY.
- IF A SERIOUS GI ADVERSE EVENT IS SUSPECTED, PROMPTLY INITIATE EVALUATION AND TREATMENT, AND DISCONTINUE NAPROXEN UNTIL A SERIOUS GI ADVERSE EVENT IS RULED OUT.
- IN THE SETTING OF CONCOMITANT USE OF LOW-DOSE ASPIRIN FOR CARDIAC PROPHYLAXIS, MONITOR PATIENTS MORE CLOSELY FOR EVIDENCE OF GI BLEEDING (SEE PRECAUTIONS; DRUG INTERACTIONS).
HEPATOTOXICITY
ELEVATIONS OF ALT OR AST (THREE OR MORE TIMES THE UPPER LIMIT OF NORMAL [ULN]) HAVE BEEN REPORTED IN APPROXIMATELY 1% OF PATIENTS IN CLINICAL TRIALS. IN ADDITION, RARE, SOMETIMES FATAL, CASES OF SEVERE HEPATIC INJURY, INCLUDING FULMINANT HEPATITIS, LIVER NECROSIS AND HEPATIC FAILURE HAVE BEEN REPORTED.
ELEVATIONS OF ALT OR AST (LESS THAN THREE TIMES ULN) MAY OCCUR IN UP TO 15% OF PATIENTS TAKING NSAIDS INCLUDING NAPROXEN.
INFORM PATIENTS OF THE WARNING SIGNS AND SYMPTOMS OF HEPATOTOXICITY (E.G., NAUSEA, FATIGUE, LETHARGY, DIARRHEA, PRURITUS, JAUNDICE, RIGHT UPPER QUADRANT TENDERNESS, AND "FLULIKE" SYMPTOMS). IF CLINICAL SIGNS AND SYMPTOMS CONSISTENT WITH LIVER DISEASE DEVELOP, OR IF SYSTEMIC MANIFESTATIONS OCCUR (E.G., EOSINOPHILIA, RASH, ETC.), DISCONTINUE NAPROXEN IMMEDIATELY, AND PERFORM A CLINICAL EVALUATION OF THE PATIENT.
HYPERTENSION
NSAIDS, INCLUDING NAPROXEN, CAN LEAD TO ONSET OF NEW HYPERTENSION OR WORSENING OF PRE-EXISTING HYPERTENSION, EITHER OF WHICH MAY CONTRIBUTE TO THE INCREASED INCIDENCE OF CV EVENTS. PATIENTS TAKING ANGIOTENSIN CONVERTING ENZYME (ACE) INHIBITORS, THIAZIDES OR LOOP DIURETICS MAY HAVE IMPAIRED RESPONSE TO THESE THERAPIES WHEN TAKING NSAIDS (SEE PRECAUTIONS; DRUG INTERACTIONS).
MONITOR BLOOD PRESSURE (BP) DURING THE INITIATION OF NSAID TREATMENT AND THROUGHOUT THE COURSE OF THERAPY.
HEART FAILURE AND EDEMA
THE COXIB AND TRADITIONAL NSAID TRIALISTS’ COLLABORATION META-ANALYSIS OF RANDOMIZED CONTROLLED TRIALS DEMONSTRATED AN APPROXIMATELY TWO-FOLD INCREASE IN HOSPITALIZATION FOR HEART FAILURE IN COX-2 SELECTIVE-TREATED PATIENTS AND NONSELECTIVE NSAID-TREATED PATIENTS COMPARED TO PLACEBO-TREATED PATIENTS. IN A DANISH NATIONAL REGISTRY STUDY OF PATIENTS WITH HEART FAILURE, NSAID USE INCREASED THE RISK OF MI, HOSPITALIZATION FOR HEART FAILURE, AND DEATH. ADDITIONALLY, FLUID RETENTION AND EDEMA HAVE BEEN OBSERVED IN SOME PATIENTS TREATED WITH NSAIDS. USE OF NAPROXEN MAY BLUNT THE CV EFFECTS OF SEVERAL THERAPEUTIC AGENTS USED TO TREAT THESE MEDICAL CONDITIONS (E.G., DIURETICS, ACE INHIBITORS, OR ANGIOTENSIN RECEPTOR BLOCKERS [ARBS]) (SEE PRECAUTIONS; DRUG INTERACTIONS).
AVOID THE USE OF NAPROXEN IN PATIENTS WITH SEVERE HEART FAILURE UNLESS THE BENEFITS ARE EXPECTED TO OUTWEIGH THE RISK OF WORSENING HEART FAILURE. IF NAPROXEN IS USED IN PATIENTS WITH SEVERE HEART FAILURE, MONITOR PATIENTS FOR SIGNS OF WORSENING HEART FAILURE.
RENAL TOXICITY AND HYPERKALEMIA
RENAL TOXICITY
LONG-TERM ADMINISTRATION OF NSAIDS HAS RESULTED IN RENAL PAPILLARY NECROSIS AND OTHER RENAL INJURY.
RENAL TOXICITY HAS ALSO BEEN SEEN IN PATIENTS IN WHOM RENAL PROSTAGLANDINS HAVE A COMPENSATORY ROLE IN THE MAINTENANCE OF RENAL PERFUSION. IN THESE PATIENTS, ADMINISTRATION OF AN NSAID MAY CAUSE A DOSEDEPENDENT REDUCTION IN PROSTAGLANDIN FORMATION AND, SECONDARILY, IN RENAL BLOOD FLOW, WHICH MAY PRECIPITATE OVERT RENAL DECOMPENSATION. PATIENTS AT GREATEST RISK OF THIS REACTION ARE THOSE WITH IMPAIRED
RENAL FUNCTION, HYPOVOLEMIA, HEART FAILURE, LIVER DYSFUNCTION, SALT DEPLETION, THOSE TAKING DIURETICS AND ACE INHIBITORS OR ARBS, AND THE ELDERLY. DISCONTINUATION OF NSAID THERAPY IS USUALLY FOLLOWED BY RECOVERY TO THE PRETREATMENT STATE. NO INFORMATION IS AVAILABLE FROM CONTROLLED CLINICAL STUDIES REGARDING THE USE OF NAPROXEN IN PATIENTS WITH ADVANCED RENAL DISEASE. THE RENAL EFFECTS OF NAPROXEN MAY HASTEN THE PROGRESSION OF RENAL DYSFUNCTION IN PATIENTS WITH PREEXISTING RENAL DISEASE.
CORRECT VOLUME STATUS IN DEHYDRATED OR HYPOVOLEMIC PATIENTS PRIOR TO INITIATING NAPROXEN. MONITOR RENAL FUNCTION IN PATIENTS WITH RENAL OR HEPATIC IMPAIRMENT, HEART FAILURE, DEHYDRATION, OR HYPOVOLEMIA DURING USE OF NAPROXEN (SEE PRECAUTIONS; DRUG INTERACTIONS). AVOID THE USE OF NAPROXEN IN PATIENTS WITH ADVANCED RENAL DISEASE UNLESS THE BENEFITS ARE EXPECTED TO OUTWEIGH THE RISK OF WORSENING RENAL FUNCTION. IF NAPROXEN IS USED IN PATIENTS WITH ADVANCED RENAL DISEASE, MONITOR PATIENTS FOR SIGNS OF
WORSENING RENAL FUNCTION.
HYPERKALEMIA
INCREASES IN SERUM POTASSIUM CONCENTRATION, INCLUDING HYPERKALEMIA, HAVE BEEN REPORTED WITH USE OF NSAIDS, EVEN IN SOME PATIENTS WITHOUT RENAL IMPAIRMENT. IN PATIENTS WITH NORMAL RENAL FUNCTION, THESE EFFECTS HAVE BEEN ATTRIBUTED TO A HYPORENINEMIC HYPOALDOSTERONISM STATE.
ANAPHYLACTOID REACTIONS
NAPROXEN HAS BEEN ASSOCIATED WITH ANAPHYLACTIC REACTIONS IN PATIENTS WITH AND WITHOUT KNOWN HYPERSENSITIVITY TO NAPROXEN AND IN PATIENTS WITH ASPIRIN-SENSITIVE ASTHMA (SEE
CONTRAINDICATIONS, WARNINGS; EXACERBATION OF ASTHMA RELATED TO ASPIRIN SENSITIVITY).
EXACERBATION OF ASTHMA RELATED TO ASPIRIN SENSITIVITY
A SUBPOPULATION OF PATIENTS WITH ASTHMA MAY HAVE ASPIRIN-SENSITIVE ASTHMA WHICH MAY INCLUDE CHRONIC RHINOSINUSITIS COMPLICATED BY NASAL POLYPS; SEVERE, POTENTIALLY FATAL BRONCHOSPASM; AND/OR INTOLERANCE TO ASPIRIN AND OTHER NSAIDS. BECAUSE CROSS-REACTIVITY BETWEEN ASPIRIN AND OTHER NSAIDS HAS BEEN REPORTED IN SUCH ASPIRIN-SENSITIVE PATIENTS, NAPROXEN TABLETS ARE CONTRAINDICATED IN PATIENTS WITH THIS FORM OF ASPIRIN SENSITIVITY (SEE CONTRAINDICATIONS). WHEN NAPROXEN TABLETS ARE USED IN PATIENTS WITH PREEXISTING ASTHMA (WITHOUT KNOWN ASPIRIN SENSITIVITY), MONITOR PATIENTS FOR CHANGES IN THE SIGNS AND SYMPTOMS OF ASTHMA.
SERIOUS SKIN REACTIONS
NSAIDS, INCLUDING NAPROXEN, CAN CAUSE SERIOUS SKIN ADVERSE EVENTS SUCH AS EXFOLIATIVE DERMATITIS, STEVENS- JOHNSON SYNDROME (SJS), AND TOXIC EPIDERMAL NECROLYSIS (TEN), WHICH CAN BE FATAL. THESE SERIOUS EVENTS MAY OCCUR WITHOUT WARNING. PATIENTS SHOULD BE INFORMED ABOUT THE SIGNS AND SYMPTOMS OF SERIOUS SKIN MANIFESTATIONS AND TO DISCONTINUE THE USE OF NAPROXEN AT THE FIRST APPEARANCE OF SKIN RASH OR ANY OTHER SIGN OF HYPERSENSITIVITY. NAPROXEN TABLETS ARE CONTRAINDICATED IN PATIENTS WITH PREVIOUS
SERIOUS SKIN REACTIONS TO NSAIDS (SEE CONTRAINDICATIONS).
PREMATURE CLOSURE OF FETAL DUCTUS ARTERIOSUS
NAPROXEN MAY CAUSE PREMATURE CLOSURE OF THE FETAL DUCTUS ARTERIOSUS. AVOID USE OF NSAIDS, INCLUDING NAPROXEN, IN PREGNANT WOMEN STARTING AT 30 WEEKS OF GESTATION (THIRD TRIMESTER) (SEE PRECAUTIONS; PREGNANCY).
HEMATOLOGIC TOXICITY
ANEMIA HAS OCCURRED IN NSAID-TREATED PATIENTS. THIS MAY BE DUE TO OCCULT OR GROSS BLOOD LOSS, FLUID RETENTION, OR AN INCOMPLETELY DESCRIBED EFFECT ON ERYTHROPOIESIS. IF A PATIENT TREATED WITH NAPROXEN HAS ANY SIGNS OR SYMPTOMS OF ANEMIA, MONITOR HEMOGLOBIN OR HEMATOCRIT.
NSAIDS, INCLUDING NAPROXEN, MAY INCREASE THE RISK OF BLEEDING EVENTS. CO-MORBID CONDITIONS SUCH AS COAGULATION DISORDERS, OR CONCOMITANT USE OF WARFARIN AND OTHER ANTICOAGULANTS, ANTIPLATELET AGENTS (E.G., ASPIRIN), SEROTONIN REUPTAKE INHIBITORS (SSRIS) AND SEROTONIN NOREPINEPHRINE REUPTAKE INHIBITORS (SNRIS) MAY INCREASE THIS RISK. MONITOR THESE PATIENTS FOR SIGNS OF BLEEDING (SEE PRECAUTIONS; DRUG
INTERACTIONS).
-
DOSAGE & ADMINISTRATION
CAREFULLY CONSIDER THE POTENTIAL BENEFITS AND RISKS OF NAPROXEN AND OTHER TREATMENT OPTIONS BEFORE DECIDING TO USE NAPROXEN TABLETS. USE THE LOWEST EFFECTIVE DOSE FOR THE SHORTEST DURATION CONSISTENT WITH INDIVIDUAL PATIENT TREATMENT GOALS (SEE WARNINGS; GASTROINTESTINAL BLEEDING, ULCERATION, AND PERFORATION).
AFTER OBSERVING THE RESPONSE TO INITIAL THERAPY WITH NAPROXEN TABLETS, THE DOSE AND FREQUENCY SHOULD BE ADJUSTED TO SUIT AN INDIVIDUAL PATIENT'S NEEDS.
DIFFERENT DOSE STRENGTHS AND FORMULATIONS (I.E., TABLETS, SUSPENSION) OF THE DRUG ARE NOT NECESSARILY BIOEQUIVALENT. THIS DIFFERENCE SHOULD BE TAKEN INTO CONSIDERATION WHEN CHANGING FORMULATION.
ALTHOUGH NAPROXEN TABLETS, NAPROXEN SUSPENSION, NAPROXEN DELAYED-RELEASED TABLETS, AND NAPROXEN SODIUM TABLETS ALL CIRCULATE IN THE PLASMA AS NAPROXEN, THEY HAVE PHARMACOKINETIC DIFFERENCES THAT MAY AFFECT ONSET OF ACTION. ONSET OF PAIN RELIEF CAN BEGIN WITHIN 1 HOUR IN PATIENTS TAKING NAPROXEN. THE RECOMMENDED STRATEGY FOR INITIATING THERAPY IS TO CHOOSE A FORMULATION AND A STARTING DOSE LIKELY TO BE EFFECTIVE FOR THE PATIENT AND THEN ADJUST THE DOSAGE BASED ON OBSERVATION OF BENEFIT AND/OR ADVERSE EVENTS. A LOWER DOSE SHOULD BE CONSIDERED IN PATIENTS WITH RENAL OR HEPATIC IMPAIRMENT OR IN ELDERLY PATIENTS (SEE WARNINGS; HEPATOTOXICITY, AND RENAL TOXICITY AND HYPERKALEMIA, AND PRECAUTIONS; GERIATRIC USE).
GERIATRIC PATIENTS
STUDIES INDICATE THAT ALTHOUGH TOTAL PLASMA CONCENTRATION OF NAPROXEN IS UNCHANGED, THE UNBOUND PLASMA FRACTION OF NAPROXEN IS INCREASED IN THE ELDERLY. CAUTION IS ADVISED WHEN HIGH DOSES ARE REQUIRED AND SOME ADJUSTMENT OF DOSAGE MAY BE REQUIRED IN ELDERLY PATIENTS. AS WITH OTHER DRUGS USED IN THE ELDERLY, IT IS PRUDENT TO USE THE LOWEST EFFECTIVE DOSE.
PATIENTS WITH MODERATE TO SEVERE RENAL IMPAIRMENT
NAPROXEN-CONTAINING PRODUCTS ARE NOT RECOMMENDED FOR USE IN PATIENTS WITH MODERATE TO SEVERE AND SEVERE RENAL IMPAIRMENT (CREATININE CLEARANCE < 30 ML/MIN) (SEE WARNINGS: RENAL EFFECTS).
RHEUMATOID ARTHRITIS, OSTEOARTHRITIS AND ANKYLOSING SPONDYLITIS
THE RECOMMENDED DOSE IS 250 MG, 375 MG, OR 500 MG TWICE DAILY. DURING LONG-TERM ADMINISTRATION, THE DOSE OF NAPROXEN MAY BE ADJUSTED UP OR DOWN DEPENDING ON THE CLINICAL RESPONSE OF THE PATIENT. A LOWER DAILY DOSE MAY SUFFICE FOR LONG-TERM ADMINISTRATION. THE MORNING AND EVENING DOSES DO NOT HAVE TO BE EQUAL IN SIZE AND THE ADMINISTRATION OF THE DRUG MORE FREQUENTLY THAN TWICE DAILY IS NOT NECESSARY. IN PATIENTS WHO TOLERATE LOWER DOSES WELL, THE DOSE MAY BE INCREASED TO NAPROXEN 1500 MG/DAY FOR LIMITED PERIODS OF UP TO 6 MONTHS WHEN A HIGHER LEVEL OF ANTI-INFLAMMATORY/ANALGESIC ACTIVITY IS REQUIRED. WHEN TREATING SUCH PATIENTS WITH NAPROXEN 1500 MG/DAY, THE PHYSICIAN SHOULD OBSERVE SUFFICIENT INCREASED CLINICAL BENEFITS TO OFFSET THE POTENTIAL INCREASED RISK. THE MORNING AND EVENING DOSES DO NOT HAVE TO BE EQUAL IN SIZE AND ADMINISTRATION OF THE DRUG MORE FREQUENTLY THAN TWICE DAILY DOES NOT GENERALLY MAKE A DIFFERENCE IN RESPONSE (SEE CLINICAL PHARMACOLOGY).
JUVENILE ARTHRITIS
NAPROXEN TABLETS MAY NOT ALLOW FOR THE FLEXIBLE DOSE TITRATION NEEDED IN PEDIATRIC PATIENTS WITH JUVENILE ARTHRITIS. A LIQUID FORMULATION MAY BE MORE APPROPRIATE. IN PEDIATRIC PATIENTS, DOSES OF 5 MG/KG/DAY PRODUCED PLASMA LEVELS OF NAPROXEN SIMILAR TO THOSE SEEN IN ADULTS TAKING 500 MG OF NAPROXEN (SEE CLINICAL PHARMACOLOGY). THE RECOMMENDED TOTAL DAILY DOSE OF NAPROXEN IS APPROXIMATELY 10 MG/KG GIVEN IN 2 DIVIDED DOSES. ONE-HALF OF THE 250 MG TABLET WILL BE NEEDED FOR DOSING LOWER-WEIGHT CHILDREN. DOSING WITH NAPROXEN TABLETS IS NOT APPROPRIATE FOR CHILDREN WEIGHING LESS THAN 25 KILOGRAMS. THE RECOMMENDED TOTAL DAILY DOSE OF NAPROXEN IS APPROXIMATELY 10 MG/KG GIVEN IN 2 DIVIDED DOSES (I.E., 5 MG/KG GIVEN TWICE A DAY). NAPROXEN TABLETS ARE NOT WELL SUITED TO THIS DOSAGE SO USE OF NAPROXEN ORAL SUSPENSION IS RECOMMENDED FOR THIS INDICATION.
MANAGEMENT OF PAIN, PRIMARY DYSMENORRHEA, AND ACUTE TENDONITIS AND BURSITIS
BECAUSE THE SODIUM SALT OF NAPROXEN IS MORE RAPIDLY ABSORBED, NAPROXEN SODIUM IS RECOMMENDED FOR THE MANAGEMENT OF ACUTE PAINFUL CONDITIONS WHEN PROMPT ONSET OF PAIN RELIEF IS DESIRED. NAPROXEN MAY ALSO BE USED. THE RECOMMENDED STARTING DOSE OF NAPROXEN IS 500 MG, FOLLOWED BY 500 MG EVERY 12 HOURS OR 250 MG EVERY 6 TO 8 HOURS AS REQUIRED. THE INITIAL TOTAL DAILY DOSE SHOULD NOT EXCEED 1250 MG OF NAPROXEN.
ACUTE GOUT
THE RECOMMENDED STARTING DOSE IS 750 MG OF NAPROXEN FOLLOWED BY 250 MG EVERY 8 HOURS UNTIL THE ATTACK HAS SUBSIDED.
-
MED GUIDE FOR NAPROXEN TABLETS USP 250 MG 375 MG 500 MG
Medication Guide for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Medication Guide for Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAIDs can cause serious side effects, including:Increased risk of a heart attack or stroke that can lead to death. This risk may happen early in treatment and may increase:
o with increasing doses of NSAIDs
o with longer use of NSAIDs
Do not take NSAIDs right before or after a heart surgery called a “coronary artery bypass graft (CABG).”
Avoid taking NSAIDs after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack.Increased risk of bleeding, ulcers, and tears (perforation) of the esophagus (tube leading from the mouth to the stomach), stomach and intestines:
o any time during use
o without warning symptoms
o that may cause death
The risk of getting an ulcer or bleeding increases with:
o past history of stomach ulcers, or stomach or intestinal bleeding with use of NSAIDs
o taking medicines called “corticosteroids”, “anticoagulants”, “SSRIs”, or “SNRIs”
o increasing doses of NSAIDs o older age
o longer use of NSAIDs o poor health
o smoking o advanced liver disease
o drinking alcohol o bleeding problems
NSAIDs should only be used:
o exactly as prescribed
o at the lowest dose possible for your treatment
o for the shortest time neededWhat are NSAIDs?
NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps, and other types of short-term pain.Who should not take NSAIDs?
Do not take NSAIDs:
• if you have had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAIDs.
• right before or after heart bypass surgery.Before taking NSAIDs, tell your healthcare provider about all of your medical conditions, including if you:
• have liver or kidney problems
• have high blood pressure
• have asthma
• are pregnant or plan to become pregnant. Talk to your healthcare provider if you are considering taking NSAIDs during pregnancy. You should not take NSAIDs after 29 weeks of pregnancy.
• are breastfeeding or plan to breastfeed.
Tell your healthcare provider about all of the medicines you take, including prescription or over-the-counter medicines, vitamins or herbal supplements. NSAIDs and some other medicines can interact with each other and cause serious side effects. Do not start taking any new medicine without talking to your healthcare provider first.What are the possible side effects of NSAIDs?
NSAIDs can cause serious side effects, including:
See “What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)"?
• new or worse high blood pressure
• heart failure
• liver problems including liver failure
• kidney problems including kidney failure
• low red blood cells (anemia)
• life-threatening skin reactions
• life-threatening allergic reactions
• Other side effects of NSAIDs include: stomach pain, constipation, diarrhea, gas, heartburn, nausea, vomiting, and dizziness.
Get emergency help right away if you have any of the following symptoms:shortness of breath or trouble breathing
chest pain
weakness in one part or side of your body
slurred speech
swelling of the face or throatStop taking your NSAID and call your healthcare provider right away if you get any of the following symptoms:
nausea
more tired or weaker than usual
diarrhea
itching
your skin or eyes look yellow
indigestion or stomach pain
flu-like symptoms
vomit blood
there is blood in your bowel movement or it is black and sticky like tar
unusual weight gain
skin rash or blisters with fever
swelling of the arms and legs, hands and feetIf you take too much of your NSAID, call your healthcare provider or get medical help right away.
These are not all the possible side effects of NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs. Call your doctor for medical advice about side effects. You may report side effects to Marksans at 1-877-376-4271 and/orFDA at 1-800-FDA-1088.Other information about NSAIDs
Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
Some NSAIDs are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.General information about the safe and effective use of NSAIDs
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which it was not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals.Manufactured for:
Time-Cap Labs, Inc.
7 Michael Avenue,
Farmingdale,
NY 11735, USA
Manufactured by:
Marksans Pharma Ltd.
Plot No. L-82, L-83,
Verna Indl. Estate,
Verna, GOA - 403722, India.This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: October 2017
- 619R - NAPROXEN 250 MG 100 COUNT
- 617R NAPROXEN 375 MG 100 COUNT
- 618R NAPROXEN 500 MG 100 COUNT
-
INGREDIENTS AND APPEARANCE
NAPROXEN
naproxen tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49483-617 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPROXEN (UNII: 57Y76R9ATQ) (NAPROXEN - UNII:57Y76R9ATQ) NAPROXEN 375 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white Score no score Shape OVAL Size 14mm Flavor Imprint Code 139 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49483-617-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/06/2016 2 NDC:49483-617-50 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/06/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091416 07/06/2016 NAPROXEN
naproxen tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49483-618 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPROXEN (UNII: 57Y76R9ATQ) (NAPROXEN - UNII:57Y76R9ATQ) NAPROXEN 500 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white Score 2 pieces Shape CAPSULE Size 16mm Flavor Imprint Code 140 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49483-618-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/06/2016 2 NDC:49483-618-50 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/06/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091416 07/06/2016 NAPROXEN
naproxen tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49483-619 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPROXEN (UNII: 57Y76R9ATQ) (NAPROXEN - UNII:57Y76R9ATQ) NAPROXEN 250 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code 138 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49483-619-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/06/2016 2 NDC:49483-619-50 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/06/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091416 07/06/2016 Labeler - TIME CAP LABORATORIES (037052099) Registrant - TIME CAP LABORATORIES (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 manufacture(49483-617, 49483-618, 49483-619)