Label: OXYGEN- oxygen gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 25373-001-01, 25373-001-02, 25373-001-03, 25373-001-04, view more25373-001-05, 25373-001-06, 25373-001-07, 25373-001-08, 25373-001-09, 25373-001-10, 25373-001-15, 25373-001-16, 25373-001-17, 25373-001-18, 25373-001-19, 25373-001-20 - Packager: Linde Gas & Equipment Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved medical gas
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 14, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
WARNINGS SECTION
WARNING: For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx only.
Uninterrupted use of high concentrations of oxygen over a long duration, without monitoring its effect on oxygen content of arterial blood, may be harmful. Do not attempt to use on patients who have stopped breathing unless used in conjunction with resuscitative equipment. Patient monitoring is recommended for patient receiving supplemental oxygen. Flow rates for this product are approximate. Federal law restricts this device to sale by or on the order of a physician.
Keep out of reach and sight of children.
Federal law requires that this container be refilled with Oxygen USP only by establishments registered as a drug manufacturer in accordance with the Federal Food, Drug, and Cosmetic Act.
Note: Keep valve minimum 12 inches (30cm) away from magnet opening. Do not move the package during MRI scan, as the MR data quality may be degraded.
-
Un-Coded Section
MR
MR CONDITIONAL
Static Field Strength 3.0T
Static Field Gradient 375 Gauss/cm
Instructions for use
-Operating temperature -4°F to 113°F (-20°C to +45°C)
-Only use with devices intended for the product administration.
-Prepare to change the cylinder when the pointer on the gauge enters the red area.
-to ensure the correct, prescribed flow rate, always make sure that the flow selector is positioned on a numbered flow setting, not between flow settings.
1) Pressure outlet (if provided)
2) Shut-off valve
3) Gauge
4) Flow outlet
5) Flow selector
Before Use:
>Check gauge (3) for content.
>Check flow selector (5) is set at zero.
>Connect equipment to outlet (1 or 4).
>Slowly open shut off valve (2) fully by turning counterclockwise.
>If equipment is connected to the flow outlet (4) select flow by turning flow selector (5) stepwise up through flow settings.
The patient can now be connected to a delivery device.
After Use:
>Turn flow selector (5) to zero by turning the flow selector stepwise down through flow settings.
>Close shut off valve (2) by turning clockwise.
>Vent downstream equipment.
>Disconnect the equipment from the outlet (1 or 4).
Batch:
DO NOT REMOVE THIS LABEL
LG015C
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

Linde
OXYGEN, COMPRESSED USP UN1072Produced By Air Liquefaction
CAS 7782-44-7
Distributed by:
Linde Gas North America LLC
200 Somerset Corporate Blvd., Suite 7000
Bridgewater, NJ 08807
866.543.3427
OXYGEN 2
MEDICAL GAS
WARNING: For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx only.
Uninterrupted use of high concentrations of oxygen over a long duration, without monitoring its effects on oxygen content of arterial blood, may be harmful. Do not attempt to use on patients who have stopped breathing unless used in conjunction with resuscitative equipment. Federal law requires that this container be refilled with Oxygen USP only by establishments registered as a drug manufacturer in accordance with the Federal Food, Drug, and Cosmetic Act.
DANGER:
KEEP OUT OF REACH OF CHILDREN.
MAY CAUSE OR INTENSIFY FIRE: OXIDIZER
CONTAINS GAS UNDER PRESSURE: MAY EXPLODE IF HEATED.
Do not handle until all safety precautions have been read and understood. Keep and store away from clothing and other combustible materials. Keep valves and fittings free from grease and oil. Use and store only outdoors or in a well-ventilated place. In case of fire: Stop leak if safe to do so. Use a back flow preventive device in the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. Open valve slowly. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52°C (125°F). Read and follow the Safety Data Sheet (SDS) before use.
DO NOT REMOVE THIS LABEL.
CONTENTS: Cylinder
TypeLiters T 8584 [_] H 6322 [_] K 6322 [_] M 3172 [_] F 1567 [_] E 680 [_] DD 588 [_] D 413 [_] C 248 [_] BB 147 [_] B 147 [_] AA 103 [_] AAA 39 [_] M-7 196 [_] LG010G

Linde
OXYGEN, COMPRESSED USPUN1072
Produced by Air Liquefaction
CAS 7782-44-7
DO NOT REMOVE THIS PRODUCT LABEL.
OXYGEN 2
LG011G
MEDICAL GAS
WARNING: For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx only. Uninterrupted use of high concentrations of oxygen over a long duration, without monitoring its effects on oxygen content or arterial blood, may be harmful. Do not attempt to use on patients who have stopped breathing unless used in conjunction with resuscitative equipment.
DANGER: MAY CAUSE OR INTENSIFY FIRE; OXIDIZER. CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED. Do not handle until all safety precautions have been read and understood. Keep and store away from clothing and other combustible materials. Keep valves and fittings free from grease and oil. Use an store only outdoors or in a well-ventilated place. In case of fire: Stop leak if safe to do so. Use a back flow preventive device in the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. Open valve slowly. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52°C (125°F). Read and follow the Safety Data Sheet (SDS) before use.
Distributed by:
Linde Gas North America LLC
200 Somerset Corporate Blvd., Suite 7000
Bridgewater, NJ 08807
866.543.3427
CONTENTS: Cylinder
TypeLiters T [_] 8584 H [_] 6322 K [_] 6322 M [_] 3172 ____ ____ ____ 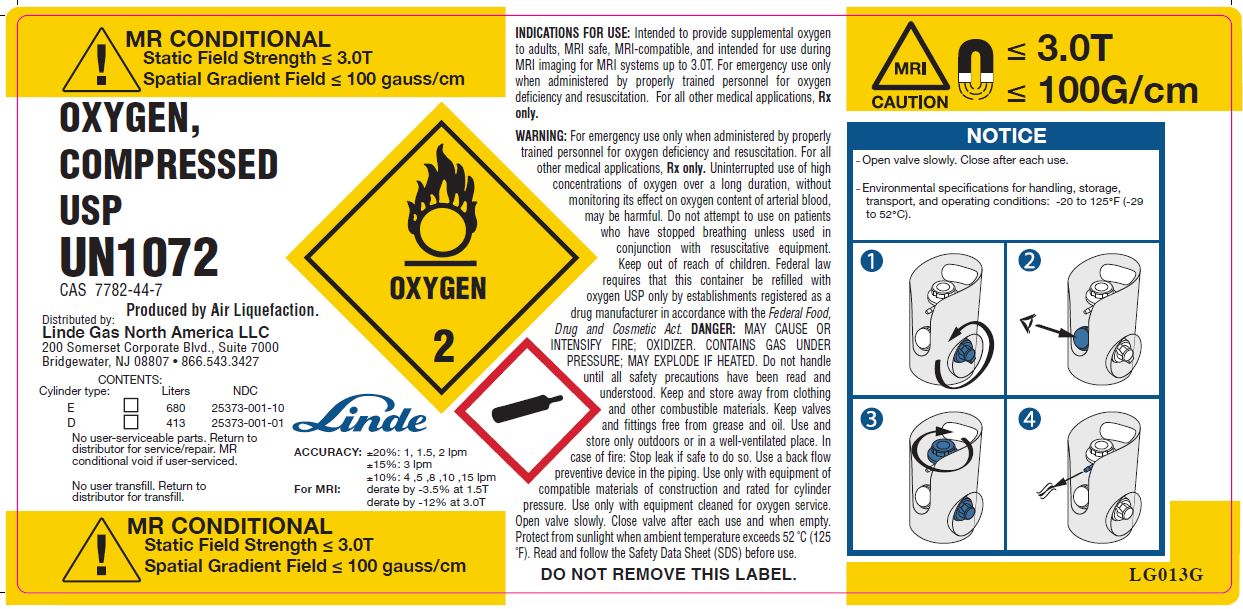
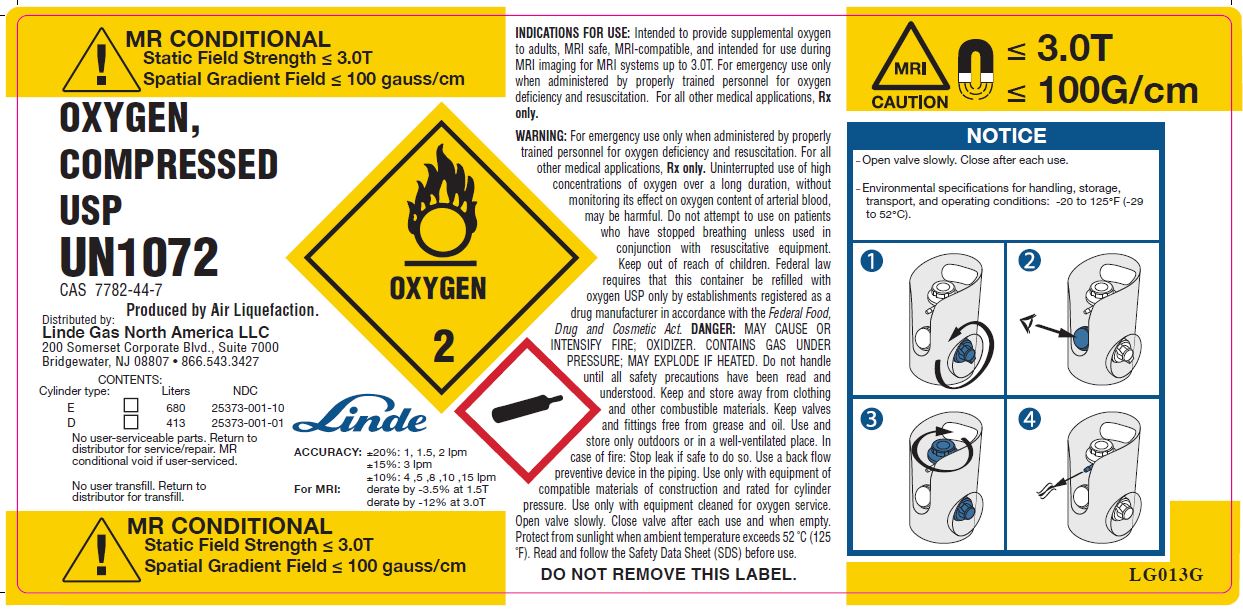
MR CONDITIONAL
Static Field Strength ≤ 3.0T
Spatial Gradient Field ≤ 100 gauss/cm
OXYGEN, COMPRESSED
USP
UN1072
CAS 7782-44-7
Produced by Air Liquefaction.
Distributed by:
Linde Gas North America, LLC
200 Somerset Corporate Blvd., Suite 7000
Bridgewater, NJ 08807
866.513.3427
CONTENTS: Cylinder Type Liters NDC E [_] 680 25373-001-10 D [_] 413 25373-001-01 No user-serviceable parts. Return to distributor for service/repair. MR conditional void if user-serviced.
No user transfill. Return to distributer for transfill.
OXYGEN 2
Linde
ACCURACY: ±20%: 1, 1.5, 2 lpm ±15%: 3 lpm ±10%: 4 ,5 ,8 ,10 ,15 lpm FOR MRI: derate by -3.5% at 1.5T derate by -12% at 3.0T MR CONDITIONAL
Static Field Strength ≤ 3.0T
Spatial Gradient Field ≤ 100 gauss/cmINDICATIONS FOR USE: Intended to provide supplemental oxygen to adults, MRI safe, MRI-compatible, and intended for use during MRI imaging for MRI systems up to 3.0T. For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx only.
WARNING: For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx only. Uninterrupted use of high concentrations of oxygen over a long duration, without monitoring its effect on oxygen content of arterial blood, may be harmful. Do not attempt to use on patients who have stopped breathing unless used in conjunction with resuscitative equipment. Keep out of reach of children. Federal law requires that this container be refilled with Oxygen USP only by establishments registered as a drug manufacturer in accordance with the Federal Food, Drug, and Cosmetic Act. DANGER: MAY CAUSE OR INTENSIFY FIRE; OXIDIZER. CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED. Do not handle until all safety precautions have been read and understood. Keep and store away from clothing and other combustible materials. Keep valves and fittings free from grease and oil. Use and store only outdoors or in a well-ventilated place. In case of fire: Stop leak if safe to do so. Use a back flow preventive device in the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. Open valve slowly. Close valve after each use and when empty. Protect from sunlight when the ambient temperature exceeds 52°C (125°F). Read and follow the Safety Data Sheet (SDS) before use.
DO NOT REMOVE THIS LABEL.
MRI
CAUTION
≤ 3.0T
≤ 100 G/cm
NOTICE
-Open valve slowly. Close after each use.
-Environmental specifications for handling, storage, transport, and operating conditions: -20 to 125°F (-29 to 52°C).
LG013G

THE LINDE GROUP
Linde
OXYGEN,
COMPRESSED
USP
OXYGEN
2
UN1072
CAS: 7782-44-7
Produced by Air Liquefaction
Oxygen (O2) 100%
under 2000 psi pressure (59°F or 15°C)
CONTENTS: Cylinder type: Liters NDC E [_] 680 25373-001-10 D [_] 413 25373-001-01 Service and transfill:
No user-serviceable parts. Return to distributor for service/repair. MRI safe and MRI compatible void if user-serviced. No user transfill. Return to distributor for transfill.
Distributed by:
Linde Gas North America LLC
200 Somerset Corporate Blvd., Suite 7000
Bridgewater, NJ 08807
866.543.3427
MR
MR CONDITIONAL
Static Field Strength 3.0T
Static Field Gradient 375 Gauss/cm
LIV® Linde Integrated Valve Portable oxygen system.
INDICATIONS FOR USE:
The LIV® is an integrated portable oxygen delivery system intended to provide supplemental oxygen to pediatrics and adults. The device is MR-conditional (per ASTM standard 2503-05), and intended for use during MR imaging for MRI systems up to 3.0T.
Rx only. Compressed gas cylinders in service or in storage shall be stabilized or otherwise secured to prevent falling or rolling.
DANGER: MAY CAUSE OR INTENSIFY FIRE; OXIDIZER. CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED.
Do not handle until all safety precautions have been read and understood. Keep and store away from clothing and other combustible materials. Keep valves and fittings free from grease and oil. Use and store only outdoors or in a well-ventilated place. In case of fire: Stop leak if safe to do so. Use a back flow preventive device in the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. Open valve slowly. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52°C (125°F). Read and follow the Safety Data Sheet (SDS) before use.
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LINDE
MEDICAL GAS
THE LINDE GROUP
Linde
OXYGEN,
COMPRESSED
USP
OXYGEN
2
UN1072
CAS: 7782-44-7
Produced by Air Liquefaction
Oxygen (O2) 100%
under 3000 psi pressure (59°F or 15°C)CONTENTS: Cylinder Type Liters NDC E [_] 1004 25373-001-15 D [_] 618 25373-001-16 Service and transfill:
No user-serviceable parts. Return to distributor for service/repair. MRI safe and MRI compatible void if user-serviced. No user transfill. Return to distributor for transfill.
Distributed by:
Linde Gas North America LLC
200 Somerset Corporate Blvd., Suite 7000
Bridgewater, NJ 08807
866.543.3427MR
MR CONDITIONAL
Static Field Strength 3.0T
Static Field Gradient 375 Gauss/cmLIV®
INDICATIONS FOR USE:
The LIV® is an integrated portable oxygen delivery system intended to provide supplemental oxygen to pediatrics and adults. The device is MR-conditional (per ASTM standard 2503-05), and intended for use during MR imaging for MRI systems up to 3.0T.
Rx only. Compressed gas cylinders in service or in storage shall be stabilized or otherwise secured to prevent falling or rolling.
DANGER: MAY CAUSE OR INTENSIFY FIRE; OXIDIZER. CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED.
Do not handle until all safety precautions have been read and understood. Keep and store away from clothing and other combustible materials. Keep valves and fittings free from grease and oil. Use and store only outdoors or in a well-ventilated place. In case of fire: Stop leak if safe to do so. Use a back flow preventive device in the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. Open valve slowly. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52°C (125°F). Read and follow the Safety Data Sheet (SDS) before use.WARNING: For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx only.
Uninterrupted use of high concentrations of oxygen over a long duration, without monitoring its effect on oxygen content of arterial blood, may be harmful. Do not attempt to use on patients who have stopped breathing unless used in conjunction with resuscitative equipment. Patient monitoring is recommended for patient receiving supplemental oxygen. Flow rates for this product are approximate. Federal law restricts this device to sale by or on the order of a physician.
Keep out of reach and sight of children.
Federal law requires that this container be refilled with Oxygen USP only by establishments registered as a drug manufacturer in accordance with the Federal Food, Drug, and Cosmetic Act.
Note: Keep valve minimum 12 inches (30cm) away from magnet opening. Do not move the package during MRI scan, as the MR data quality may be degraded.
DO NOT REMOVE THIS LABEL
MR
MR CONDITIONAL
Static Field Strength 3.0T
Static Field Gradient 375 Gauss/cm
Instructions for use
-Operating temperature -4°F to 113°F (-20°C to +45°C)
-Only use with devices intended for the product administration.
-Prepare to change the cylinder when the pointer on the gauge enters the red area.
-to ensure the correct, prescribed flow rate, always make sure that the flow selector is positioned on a numbered flow setting, not between flow settings.
1) Pressure outlet (if provided)
2) Shut-off valve
3) Gauge
4) Flow outlet
5) Flow selector
Before Use:
>Check gauge (3) for content.
>Check flow selector (5) is set at zero.
>Connect equipment to outlet (1 or 4).
>Slowly open shut off valve (2) fully by turning counterclockwise.
>If equipment is connected to the flow outlet (4) select flow by turning flow selector (5) stepwise up through flow settings.
The patient can now be connected to a delivery device.
After Use:
>Turn flow selector (5) to zero by turning the flow selector stepwise down through flow settings.
>Close shut off valve (2) by turning clockwise.
>Vent downstream equipment.
>Disconnect the equipment from the outlet (1 or 4).
Batch:
LG016B
THE LINDE GROUP
Linde
OXYGEN,
COMPRESSED
USP
OXYGEN
2
UN1072
CAS: 7782-44-7
Produced by Air Liquefaction
Oxygen (O2) 100%
under 2000 psi pressure (59°F or 15°C)CONTENTS: Cylinder type: Liters NDC E [_] 680 25373-001-10 D [_] 413 25373-001-01 Service and transfill:
No user-serviceable parts. Return to distributor for service/repair. MRI safe and MRI compatible void if user-serviced. No user transfill. Return to distributor for transfill.
Federal law requires that this container be refilled with oxygen USP only by establishments registered as a drug manufacturer in accordance with the Federal Food, Drug, and Cosmetic Act.
DO NOT REMOVE THIS LABEL
Distributed by:
Linde Gas North America LLC
200 Somerset Corporate Blvd., Suite 7000
Bridgewater, NJ 08807
866.543.3427Note: Keep valve minimum 12 inches (30 cm) away from magnet opening. Do not move the package during MRI scan, as the MR data quality may be degraded.
MR
MR CONDITIONAL
Static Field Strength 3.0T
Static Field Gradient 375 Gauss/cmLIV®IQ Linde Integrated Valve Portable oxygen system
INDICATIONS FOR USE:
The LIV®IQ is an integrated portable oxygen delivery system intended to provide supplemental oxygen to pediatrics and adults. The device is MR-conditional (per ASTM standard 2503-05), and intended for use during MR imaging for MRI systems up to 3.0T.
Rx only. Compressed gas cylinders in service or in storage shall be stabilized or otherwise secured to prevent falling or rolling.
DANGER: MAY CAUSE OR INTENSIFY FIRE; OXIDIZER. CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED.
Do not handle until all safety precautions have been read and understood. Keep and store away from clothing and other combustible materials. Keep valves and fittings free from grease and oil. Use and store only outdoors or in a well-ventilated place. In case of fire: Stop leak if safe to do so. Use a back flow preventive device in the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. Open valve slowly. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52°C (125°F). Read and follow the Safety Data Sheet (SDS) before use.WARNING: For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx only.
Uninterrupted use of high concentrations of oxygen over a long duration, without monitoring its effect on oxygen content of arterial blood, may be harmful. Do not attempt to use on patients who have stopped breathing unless used in conjunction with resuscitative equipment. Patient monitoring is recommended for patient receiving supplemental oxygen. Flow rates for this product are approximate. Federal law restricts this device to sale by or on the order of a physician.
Keep out of reach and sight of children.Download the full user guide at: www.liv-iq.com or scan with your smart phone:
Guide to Digital Display
Selected Flow
Flow is active
New, full cylinder
Therapy time remaining at selected flow setting
Remaining gas volume, shown 3/4 full
Mute button
If there are no lines or numbers around the content indicator, do not use the product.
MR
MR CONDITIONAL
Static Field Strength 3.0T
Static Field Gradient 375 Gauss/cm
Instructions for use
-Operating temperature -4°F to 113°F (-20°C to +45°C)
-Only use with devices intended for the product administration.
-Prepare to change the cylinder when the pointer on the gauge enters the red area.
-to ensure the correct, prescribed flow rate, always make sure that the flow selector is positioned on a numbered flow setting, not between flow settings.
1) Pressure outlet (if provided)
2) Shut-off valve
3) Flow selector
4) Flow outlet
5) Digital display
Mute button
Before Use:
>Check digital display (5) for content.
>Check flow selector (3) is set at zero.
>Connect equipment to outlet (1 or 4).
>Slowly open shut off valve (2) slowly to "ON".
>Turn the flow selector (3) to the desired flow.
The patient can now be connected to a delivery device.
After Use:
>Remove the delivery device from the patient.
>Turn flow selector (3) to zero.
>Turn the shut off valve (2) to "OFF".
>Turn the flow selector (3) to a high flow to vent.
>Turn the flow selector (3) back to zero.
>Disconnect the delivery device from outlet (1 or 4).
-Using both outlets at the same time is not recommended and may cause a "HI FLO" alarm. To silence the alarm and continue, press and hold the mute button for 3 seconds.
-The mute button can silence any alarm for 1 minute (short press) or permanently (press & hold for 3 seconds).
Set flow selector to zero before opening shut-off valve.
Blocked tube/Low flow
Has the Mute button been pressed?
Yes: An active alarm has been muted.
No: Due to a rapid change in temperature, the low flow or high flow alert is temporarily disabled.
Batch:
LGIQ015B
-
INGREDIENTS AND APPEARANCE
OXYGEN
oxygen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:25373-001 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Oxygen (UNII: S88TT14065) (Oxygen - UNII:S88TT14065) Oxygen 99 L in 100 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25373-001-01 413 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 2 NDC:25373-001-02 640 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 3 NDC:25373-001-03 6452 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 4 NDC:25373-001-04 34 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 5 NDC:25373-001-05 113 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 6 NDC:25373-001-06 170 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 7 NDC:25373-001-07 170 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 8 NDC:25373-001-08 196 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 9 NDC:25373-001-09 248 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 10 NDC:25373-001-10 680 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 11 NDC:25373-001-17 1567 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 12 NDC:25373-001-18 3172 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 13 NDC:25373-001-19 6322 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 14 NDC:25373-001-20 8584 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 15 NDC:25373-001-15 1004 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 16 NDC:25373-001-16 618 L in 1 CYLINDER; Type 0: Not a Combination Product 11/19/1990 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved medical gas 11/19/1990 Labeler - Linde Gas & Equipment Inc. (805568339) Registrant - Linde Gas & Equipment Inc. (805568339) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 010263301 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 016271697 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 833132561 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 063018874 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 069239874 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 784579190 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 016300933 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 164139060 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 026921048 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 129012352 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 142591424 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 168248081 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 067906953 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 789082976 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 149324605 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 833120244 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 833120723 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 134971428 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 839176356 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 069232473 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 160443631 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 833132108 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 080006834 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 080428082 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 019551467 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 078692645 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 181444840 manufacture(25373-001) Establishment Name Address ID/FEI Business Operations Linde Gas & Equipment Inc. 805823411 manufacture(25373-001)