Label: OXYGEN gas
-
NDC Code(s):
10451-001-01,
10451-001-02,
10451-001-03,
10451-001-04, view more10451-001-05, 10451-001-06, 10451-001-07, 10451-001-08, 10451-001-09, 10451-001-10, 10451-001-11, 10451-001-12, 10451-001-13
- Packager: General Distributing Co.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
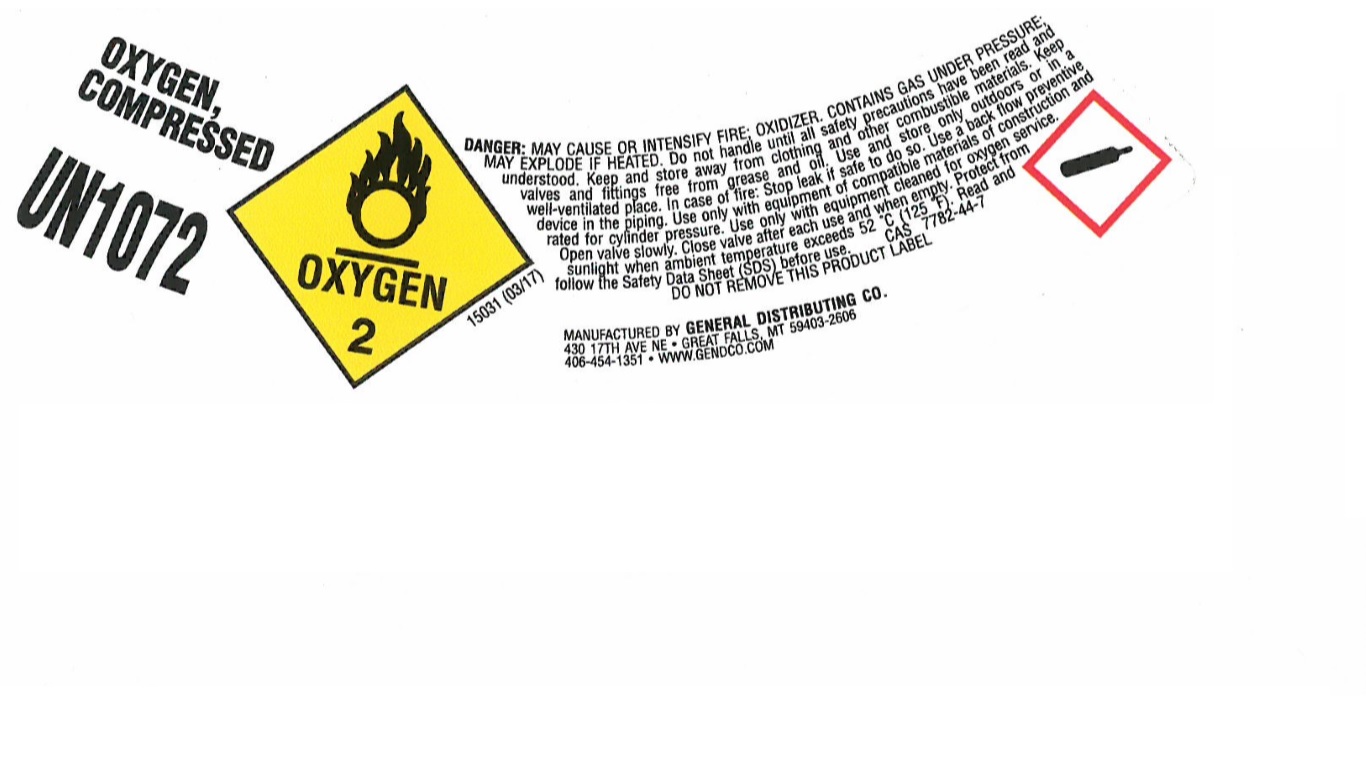
PRINCIPAL DISPLAY PANEL
OXYGEN, COMPRESSED
UN1072
DANGER: MAY CAUSE OR INTENSIFY FIRE; OXIDIZER. CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED. Do not handle until all safety precautions have been red and understood. Keep and store away from clothing and other combustible materials. Keep valves and fitting free from grease and oil. Use and store only outdoors or in a well-ventilated place. In case of fire: Stop leak if safe to do so. Use a back flow preventive device in the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. Open valve slowly. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52 C (125 F). Read and follow the Safety Data Sheet (SDS) before use. CAS 7782-44-7
DO NOT REMOVE THIS PRODUCT LABEL
MANUFACTURED BY GENERAL DISTRIBUTING CO.
430 17TH AVE NE
GREAT FALLS, MY 59403-2606
406-454-1351
WWW.GENDCO.COM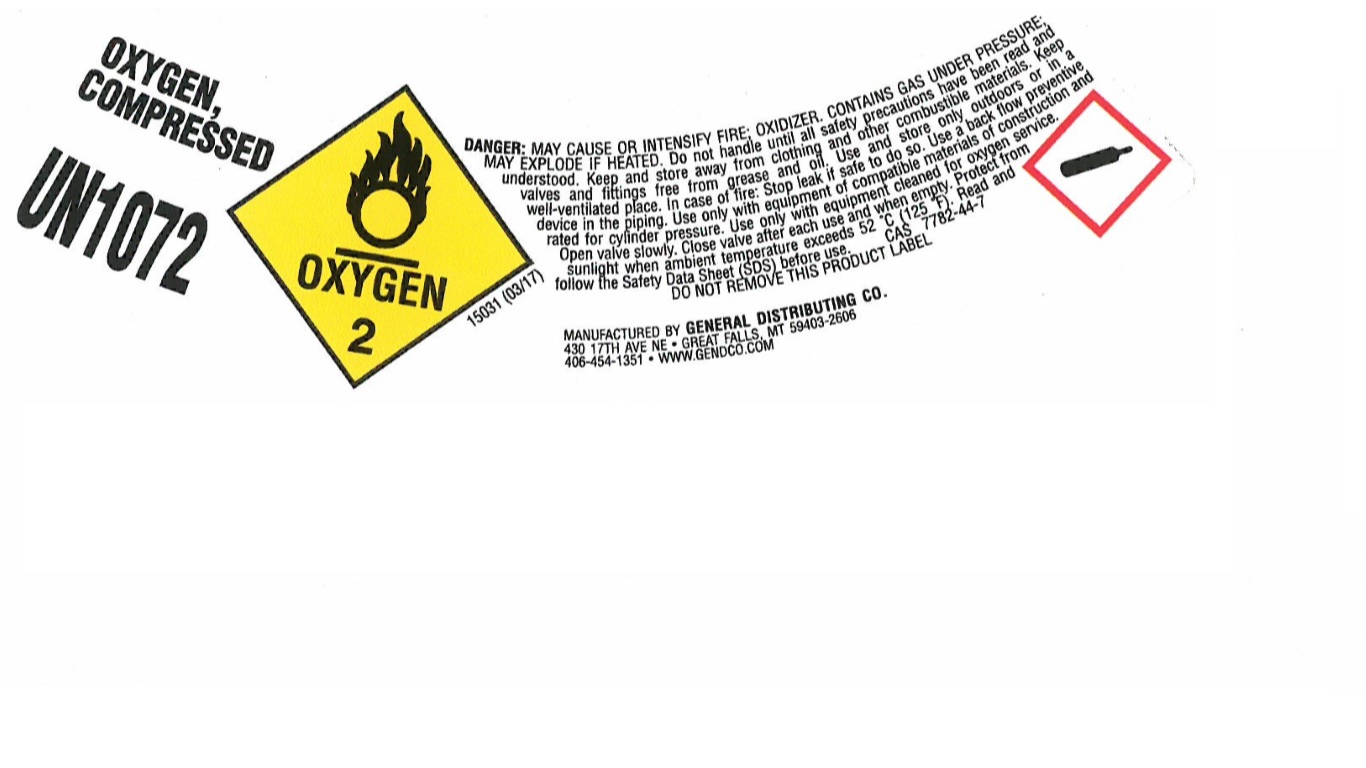
-
INGREDIENTS AND APPEARANCE
OXYGEN
oxygen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10451-001 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYGEN (UNII: S88TT14065) (OXYGEN - UNII:S88TT14065) OXYGEN 992 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10451-001-01 663 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 2 NDC:10451-001-02 408 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 3 NDC:10451-001-03 120 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 4 NDC:10451-001-04 129621 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 5 NDC:10451-001-05 4350 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 02/01/2022 6 NDC:10451-001-06 5050 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 02/01/2022 7 NDC:10451-001-07 3511 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 8 NDC:10451-001-08 2350 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 9 NDC:10451-001-09 143 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 10 NDC:10451-001-10 158 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 11 NDC:10451-001-11 174 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 12 NDC:10451-001-12 7079 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 13 NDC:10451-001-13 8495 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/1969 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205889 01/01/1969 Labeler - General Distributing Co. (006241236) Establishment Name Address ID/FEI Business Operations General Distributing Co. 006241236 manufacture(10451-001) Establishment Name Address ID/FEI Business Operations General Distributing Company 035223452 manufacture(10451-001) Establishment Name Address ID/FEI Business Operations General Distributing Company 051674617 manufacture(10451-001) Establishment Name Address ID/FEI Business Operations General Distributing Co 078806971 manufacture(10451-001) Establishment Name Address ID/FEI Business Operations General Distributing Company 079713202 manufacture(10451-001) Establishment Name Address ID/FEI Business Operations General Distributing Company 840608442 manufacture(10451-001)