Label: CYFENDUS- anthrax vaccine adsorbed, adjuvanted injection, suspension
- NDC Code(s): 71665-001-01, 71665-001-02
- Packager: Emergent Product Development Gaithersburg Inc.
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated September 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CYFENDUS safely and effectively. See full prescribing information for CYFENDUS.
CYFENDUS™ (Anthrax Vaccine Adsorbed, Adjuvanted)
Suspension for Intramuscular Injection
Initial U.S. Approval: 2023INDICATIONS AND USAGE
CYFENDUS (Anthrax Vaccine Adsorbed, Adjuvanted) is a vaccine indicated for post-exposure prophylaxis of disease following suspected or confirmed exposure to Bacillus anthracis in persons 18 through 65 years of age when administered in conjunction with recommended antibacterial drugs.
The efficacy of CYFENDUS for post-exposure prophylaxis is based solely on studies in animal models of inhalational anthrax. (1)DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Suspension for injection; each dose is 0.5 mL. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
Appropriate medical treatment must be available to manage possible anaphylactic reactions following administration of CYFENDUS. (5.1)
CYFENDUS can cause fetal harm when administered to a pregnant individual. In an observational study, there were more birth defects in infants born to individuals vaccinated with BioThrax (a licensed anthrax vaccine with the same active ingredient as CYFENDUS) in the first trimester compared to infants born to individuals vaccinated post pregnancy or individuals never vaccinated with BioThrax. (5.3)ADVERSE REACTIONS
The most common (≥10%) injection-site adverse reactions reported were tenderness (88.1%), pain (86.3%), arm motion limitation (63.7%), warmth (51.2%), induration (37.5%), itching (21.9%), swelling (19.7%), and erythema/redness (17.9%). The most common systemic adverse reactions (≥10%) were muscle aches (75.2%), tiredness (67.1%) and headache (58.0%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Emergent BioSolutions at 1-800-768-2304 or medicalinformation@ebsi.com or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
5.2 Altered Immunocompetence
5.3 Pregnancy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Post-Exposure Prophylaxis
14.2 Concomitant Administration with Ciprofloxacin or Doxycycline
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
CYFENDUS (Anthrax Vaccine Adsorbed, Adjuvanted) is a vaccine indicated for post-exposure prophylaxis of disease following suspected or confirmed exposure to Bacillus anthracis in persons 18 through 65 years of age when administered in conjunction with recommended antibacterial drugs.
The efficacy of CYFENDUS for post-exposure prophylaxis (PEP) is based solely on studies in animal models of inhalational anthrax.
-
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
2.1 Dose and Schedule
Administer CYFENDUS by intramuscular injection as a series of two doses (0.5 mL each) two weeks apart (at Week 0 and 2) post-exposure combined with antibacterial therapy.
2.2 Administration
Gently swirl or roll the vial to ensure that the vaccine is a homogeneous milky-white suspension. To avoid foaming DO NOT shake.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, do not administer the vaccine.
Administer CYFENDUS intramuscularly.
Do not mix CYFENDUS with any other product in the same syringe or vial.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer CYFENDUS to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) following a previous dose of CYFENDUS, BioThrax or any component of the vaccine [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment must be available to manage possible anaphylactic reactions following administration of CYFENDUS.
5.2 Altered Immunocompetence
Immunocompromised persons, including those receiving immunosuppressive therapy, may have a diminished immune response to CYFENDUS.
5.3 Pregnancy
CYFENDUS can cause fetal harm when administered to a pregnant individual. In an observational study, there were more birth defects in infants born to individuals vaccinated with BioThrax (a licensed anthrax vaccine with the same active ingredient as CYFENDUS; BioThrax does not contain CPG 7909 adjuvant) in the first trimester compared to infants born to individuals vaccinated post pregnancy or individuals never vaccinated with BioThrax. [See Use in Specific Populations (8.1)]
If CYFENDUS is administered during pregnancy, the vaccinated individual should be apprised of the potential hazard to a fetus.
-
6 ADVERSE REACTIONS
The most common (≥10%) injection-site adverse reactions reported were tenderness (88.1%), pain (86.3%), arm motion limitation (63.7%), warmth (51.2%), induration (37.5%), itching (21.9%), swelling (19.7%), and erythema/redness (17.9%.). The most common systemic adverse reactions (≥10%) were muscle aches (75.2%), tiredness (67.1%), and headache (58.0%) (see Table 1).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in clinical practice.
The safety of CYFENDUS was evaluated in 4 clinical studies, in which a total of 3,276 participants 18 through 65 years of age received at least one dose of CYFENDUS and were included in a pooled safety population.
Study 1 (NCT01263691) evaluated the safety and immunogenicity of different vaccine formulations administered intramuscularly two weeks apart in participants between 18 and 50 years of age. Seventeen participants received at least one 0.5 mL dose of CYFENDUS.
Study 2 (NCT01770743) evaluated the safety and immunogenicity of different dosing regimens of CYFENDUS in participants between 18 and 50 years of age. Forty-four participants received two doses of CYFENDUS via the intramuscular route two weeks apart.
Study 3 (NCT04067011) investigated potential interactions of intramuscularly administered CYFENDUS with the antibacterials ciprofloxacin or doxycycline, when administered concomitantly in participants between 18 and 45 years of age. Sixty-four participants received only CYFENDUS in the control arm.
Study 4 (NCT03877926) evaluated the safety and immunogenicity of CYFENDUS in participants between 18 and 65 years of age. CYFENDUS was administered intramuscularly as two doses at Weeks 0 and 2, with saline placebo given at Week 4. BioThrax was administered subcutaneously as three doses at Weeks 0, 2, and 4. The number of participants receiving at least one dose of CYFENDUS was 3151, while 2898 participants received the complete two dose regimen. [See Clinical Studies (14.1)]
The mean age for the pooled safety population across Studies 1-4 was 39 years; 32.1% of participants (n=1050) were between 18-30 years of age, 44.2% (n=1447) were between 31 to 50 years of age, and 23.8% (n=779) were between 51-65 years of age. Females comprised 57.8% (n=1895) of the population, with 40.4% (n=1325) being women of childbearing potential. The race distribution was as follows: 77.9% White, 17.1% Black or African American, 1.8% Asian, 1.7% Multiracial, 0.4% American Indian or Alaskan Native, 0.3% Native Hawaiian or Other Pacific Islander, 0.5% Other, and 0.2% Unknown. Most participants (83.9%) were not Hispanic or Latino.
Study 4 Solicited Local and Systemic Adverse Reactions
In Study 4, an active-controlled study, the licensed anthrax vaccine, BioThrax (Anthrax Vaccine Adsorbed), was used as the comparator. Solicited local and systemic adverse reactions reported following administration of any dose of CYFENDUS or BioThrax are presented in Table 1. CYFENDUS was administered intramuscularly as two doses at Weeks 0 and 2, with saline placebo given at Week 4. BioThrax was administered subcutaneously as three doses at Weeks 0, 2, and 4. The number of participants receiving at least one dose of CYFENDUS was 3151, while 2898 participants received the complete two dose regimen of CYFENDUS. There was no notable difference in the frequency of solicited local or systemic adverse reactions after the first or second dose of CYFENDUS, with the exception of itching (10.3% after first CYFENDUS dose vs. 16.8% after second dose), erythema/redness (7.4% after first CYFENDUS dose vs. 14.8% after second dose), swelling (10.2% after first CYFENDUS dose vs. 15.9% after second dose) and fever (2.7% after first CYFENDUS dose vs. 5.0% after second dose).
Table 1 Percentage (%) of Participants with Solicited Local or Systemic Adverse Reactions within 7 Days Following Administration of Any Vaccine Dosea in Study 4b a 3151 participants received at least one dose, and 2898 participants received two doses of CYFENDUS. b Percentage of participants reporting solicited local or systemic adverse reactions in e-diaries and during in-clinic reactogenicity assessments for at least 7 days following any dose (CYFENDUS up to two doses intramuscularly, BioThrax up to three doses subcutaneously). c No Grade 4 local adverse reactions were reported for CYFENDUS. For all local adverse reactions, Grade 3 included a criterion that the symptom prevents activities of daily living or requires treatment. Some local adverse reactions included additional or alternative Grade 3 criteria as noted in the respective footnotes. d Pain: Grade 3 = Pain requires use of narcotic pain reliever or prevents daily activity; Grade 4 = Emergency Room visit or hospitalization. e Induration/swelling: Any Grade = ≥2.5 cm and does not interfere with activity; Grade 3 = >10 cm or prevents daily activity; Grade 4 = Necrosis. f Erythema/redness: Any Grade = ≥2.5 cm; Grade 3 = >10 cm; Grade 4 = Necrosis or exfoliative dermatitis. g No Grade 4 systemic adverse reactions were reported for CYFENDUS. Systemic adverse event: Grade 1 = Mild; Grade 2 = Moderate; Grade 3 = Severe (symptom prevents activities of daily living or requires treatment); Grade 4 = Severe (potentially life threatening). h Fever (oral temperature): Grade 1 = 38.0-38.4ᵒC; Grade 2 = 38.5-38.9ᵒC; Grade 3 = 39.0-40ᵒC; Grade 4 = >40ᵒC. CYFENDUS
CYFENDUS
CYFENDUS
BioThrax
BioThrax
BioThrax
N
Any Grade
Grade 3+
N
Any Grade
Grade 3+
Local Adverse Reactionsc
Any Injection Site Reaction
3106
93.0%
3.8%
527
94.9%
4.6%
Tenderness
3106
88.1%
1.7%
527
89.9%
0.8%
Paind
3106
86.3%
2.1%
527
87.9%
0.9%
Arm Motion Limitation
3106
63.7%
1.7%
527
51.4%
0.4%
Warmth
3106
51.2%
0.7%
527
68.7%
0.2%
Induratione
3106
37.5%
0.3%
527
75.5%
1.1%
Itching
3106
21.9%
0.4%
527
58.8%
0.8%
Swellinge
3106
19.7%
0.4%
527
55.4%
1.3%
Erythema/ Rednessf
3106
17.9%
0.9%
527
53.9%
1.9%
Bruising
3106
17.2%
0.3%
527
34.9%
0.0%
Systemic Adverse Reactionsg
Any Systemic Reaction
3115
84.3%
6.6%
528
78.4%
3.8%
Muscle Ache
3106
75.2%
3.5%
527
63.4%
1.9%
Tiredness
3106
67.1%
2.9%
527
53.7%
1.7%
Headache
3106
58.0%
3.2%
527
47.6%
2.1%
Feverh
3113
6.8%
0.7%
527
1.7%
0.4%
Serious Adverse Events (SAEs) and Deaths
In the pooled safety population, serious adverse events including deaths were monitored for up to one year following the last vaccination. None of the reported deaths (n=6, 0.2%) or SAEs (n=62, 1.9%) were determined to be related to the administration of CYFENDUS.
Potentially Immune-mediated Adverse Events
In the pooled safety population, participants were monitored for the occurrence of new-onset potentially immune-mediated adverse events for 12 months after the first dose of vaccine. Events were adjudicated as to whether they were of autoimmune etiology by an external expert blinded to treatment assignment. As determined by the adjudicator, three events of autoimmune etiology in three participants were considered possibly related to the administration of CYFENDUS: (1) a case of ulcerative colitis that occurred 208 days after the administration of CYFENDUS, (2) a case of chronic idiopathic urticaria that occurred 76 days after the administration of CYFENDUS and (3) a case of diffuse alopecia that occurred 17 days after the administration of CYFENDUS.
6.2 Postmarketing Experience
There is no postmarketing experience following administration of CYFENDUS. However, the postmarketing safety experience with BioThrax is relevant because BioThrax and CYFENDUS contain the same active ingredient and are manufactured similarly. BioThrax does not contain CPG 7909 adjuvant.
The following adverse events have been spontaneously reported during the postmarketing use of BioThrax and may occur in people receiving CYFENDUS. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. Adverse events included below are listed due to one or more of the following factors: (1) seriousness of the event, (2) number of reports, or (3) strength of causal relationship to BioThrax.
- •
- Blood and lymphatic system disorders: Lymphadenopathy
- •
- Gastrointestinal disorders: Nausea
- •
- Immune system disorders: Allergic reactions (including anaphylaxis, angioedema, rash, urticaria, pruritus, erythema multiforme, anaphylactoid reaction, and Stevens-Johnson syndrome)
- •
- Nervous system disorders: Paresthesia, syncope, dizziness, tremor, ulnar nerve neuropathy
- •
- Musculoskeletal, connective tissue, and bone disorders: Arthralgia, arthropathy, myalgia, rhabdomyolysis, alopecia
- •
- General disorders and administration site conditions: Malaise, pain, cellulitis, flu-like symptoms
- •
- Psychiatric disorders: Insomnia
- •
- Skin and subcutaneous tissue disorders: Pruritus, rash, urticaria
- •
- Vascular disorders: Flushing
Reports of the following were also received: multisystem disorder defined as chronic symptoms involving at least two of the following three categories: fatigue, mood-cognition, and musculoskeletal system.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In clinically recognized pregnancies in the US general population, the estimated background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20%.
There are no adequate and well-controlled studies of CYFENDUS in pregnant individuals.
Available human data on CYFENDUS administered to pregnant individuals do not establish the presence or absence of vaccine-associated risks in pregnancy (see Human Data). However, available data on BioThrax (a licensed anthrax vaccine), administered to pregnant individuals are relevant to CYFENDUS because BioThrax and CYFENDUS contain the same active ingredient and are manufactured similarly. BioThrax does not contain CPG 7909 adjuvant. Data are available from a BioThrax observational study and pregnancy exposure registry.
In the observational study there were more birth defects in infants born to individuals vaccinated with BioThrax in the first trimester compared to individuals vaccinated post pregnancy or individuals never vaccinated with BioThrax. Data from the BioThrax pregnancy exposure registry do not establish the presence or absence of vaccine-associated risks in pregnancy (see Human Data).
In a developmental study with an embryo-fetal development toxicity phase, female rats were administered a full human dose (0.5 mL) of CYFENDUS twice prior to mating and once during gestation. This study revealed no evidence of harm to the fetus, changes in reproductive performance, or adverse effects on post-natal development due to the vaccine (see Animal Data).
Data
In pre-licensure clinical studies of CYFENDUS, women underwent pregnancy testing immediately prior to administration of each dose of CYFENDUS. Despite this pregnancy screening regimen, some subjects were vaccinated with CYFENDUS very early in pregnancy before human chorionic gonadotropin was detectable (n=10) or in the 30 days prior to pregnancy onset (n=1). Of the 11 pregnancies (one twin pregnancy), 1 (9.1%) resulted in miscarriage and there were 2 infants (18.2%) born with major birth defects.
An observational study examined the rate of birth defects among 37,140 infants born to US military service personnel who received BioThrax during pregnancy between 1998 and 2004. In this study, birth defects were slightly more common in first trimester-exposed infants (4.68%) when compared with infants of individuals vaccinated post pregnancy (3.85%) (odds ratio = 1.20; 95% confidence interval: 1.005, 1.43) or when compared to individuals never vaccinated with BioThrax (4.03%) (odds ratio = 1.20; 95% confidence interval: 1.02, 1.42)1.
A pregnancy exposure registry was conducted in individuals who received BioThrax. Of 91 individuals who reported pregnancy outcomes, the majority of exposures were in the first trimester (n=89), and there were two infants with major birth defects (2.2%) and no miscarriages.
In a pre- and post-natal developmental study with an embryo-fetal development toxicity phase performed in female rats, a full human dose (0.5 mL) of CYFENDUS was administered by intramuscular injection on three occasions: 14 days prior to start of cohabitation, on the day of cohabitation, and on Gestation Day 7. No vaccine-related adverse effects on fetal development, reproductive performance, or pre- and post-natal development up to post-natal day 21 in the offspring were reported.
8.2 Lactation
Risk Summary
It is not known whether CYFENDUS is excreted in human milk. Human data are not available to assess the impact of the vaccine on milk production, its presence in breast milk, or its effects on the breastfed child. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for CYFENDUS and any potential adverse effects on the breastfed child, or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
-
11 DESCRIPTION
CYFENDUS (Anthrax Vaccine Adsorbed, Adjuvanted) is a sterile, milky-white suspension for intramuscular administration.
CYFENDUS is made from cell-free filtrates of microaerophilic cultures of an avirulent, nonencapsulated strain of B. anthracis. The production cultures are grown in a chemically defined protein-free medium consisting of a mixture of amino acids, vitamins, inorganic salts, and sugars. CYFENDUS is prepared from sterile culture filtrates containing proteins released during growth, including the 83-kDa protective antigen protein, and contains no dead or live bacteria. The sterile culture filtrates containing the proteins are adsorbed onto aluminum hydroxide adjuvant and combined with a saline preservative solution containing the excipients sodium chloride, formaldehyde, benzethonium chloride, and water for injection. CPG 7909 adjuvant is added to the preservative solution containing the adsorbed proteins to form CYFENDUS.
CPG 7909 adjuvant is a synthetic DNA molecule 24 nucleotides in length with a nuclease-resistant phosphorothioate backbone.
CYFENDUS contains 100 mcg/mL total adsorbed cell-free filtrate, 1.3 mg/mL aluminum adjuvant, 0.5 mg/mL CPG 7909 adjuvant, and 0.85% sodium chloride, with 25 mcg/mL benzethonium chloride and 100 mcg/mL formaldehyde added as preservatives. A single dose of CYFENDUS is 0.5 mL.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Anthrax is an infectious disease caused by the Gram-positive, spore-forming bacterium B. anthracis.
CYFENDUS induces antibodies against protective antigen protein that may contribute to protection by neutralizing the activities of the cytotoxic lethal toxin and edema toxin of B. anthracis.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
CYFENDUS has not been evaluated for carcinogenicity, mutagenic potential, or male infertility in animals. CYFENDUS administered to female rats had no effect on fertility [see Use in Specific Populations (8.1)].
13.2 Animal Toxicology and/or Pharmacology
Animal Pharmacology
Because it is not feasible or ethical to conduct controlled clinical trials with anthrax, the efficacy of CYFENDUS is based on animal studies with CYFENDUS and BioThrax. Pre-exposure prophylaxis studies conducted in animal models were used to derive protective antibody thresholds to bridge animal efficacy and human immunogenicity data and predict efficacy in humans.
Pivotal efficacy animal studies with BioThrax were conducted in rabbits and nonhuman primates (NHPs). Animals received two intramuscular vaccinations four weeks apart with serial dilutions of BioThrax and were subjected to lethal challenge on study day 70 with aerosolized B. anthracis spores at a target dose exceeding the 50% lethal dose by 200-fold. Serum samples were collected at various time points prior to challenge for immune response analysis via anthrax lethal toxin neutralizing antibody (TNA) assay. The relationship between pre-challenge serum TNA levels and survival was evaluated. Logistic regression analysis demonstrated that a 70% probability of survival was associated with a TNA NF50 (50% neutralization factor) level of 0.56 in rabbits and 0.29 in NHPs.
Efficacy studies for CYFENDUS were conducted in guinea pigs and cynomolgus macaques. Animals received two intramuscular vaccinations two weeks apart with various dilutions of CYFENDUS and were challenged with a lethal dose of aerosolized B. anthracis spores on Day 28 or 70. The studies demonstrated a strong correlation between pre-challenge serum TNA levels and survival.
The ability of CYFENDUS to increase survival after the cessation of the post-exposure antibacterial treatment, as compared with antibacterial treatment alone, was investigated in a guinea pig post-exposure prophylaxis study. In this study, guinea pigs were challenged with a lethal dose of aerosolized B. anthracis spores on Day 0 and treated with ciprofloxacin (7.5 mg/kg three times daily) for two weeks after the challenge. Various dilutions of the vaccine were administered on Days 1 and 8 after the challenge. Post-exposure vaccination increased animal survival in the 21-day period following cessation of antibacterial drugs, compared to antibacterial treatment alone, in a dose-dependent manner.
-
14 CLINICAL STUDIES
14.1 Post-Exposure Prophylaxis
The efficacy of CYFENDUS is based on studies of both CYFENDUS and BioThrax in animals [see Animal Toxicology and/or Pharmacology (13.2)].
Based on the animal model-derived TNA thresholds from studies of BioThrax, a multicenter, randomized, active-controlled, double-blind, parallel-group clinical study (Study 4; NCT03877926) was conducted to evaluate the immunogenicity and safety of a post-exposure administration schedule of two doses (0.5 mL) of CYFENDUS intramuscularly two weeks apart in adults ages 18 through 65 years. In the study, 3151 participants received at least one dose of CYFENDUS, and 533 participants received at least one dose (0.5 mL) of the comparator licensed anthrax vaccine, BioThrax. Participants were followed for immunogenicity up to Day 64 (7 weeks after last CYFENDUS vaccination, 5 weeks after last BioThrax vaccination) [see Adverse Reactions (6.1)].
The mean age of participants in the per protocol (PP) population receiving CYFENDUS (n=2543) was 39.4 years. Of these, 57.8% of participants were female, 78.1% were White, 17.0% were Black or African American, 1.7% were Multiracial, 1.6% were Asian, 0.5% were Other, 0.4% were American Indian or Alaskan Native, and 0.4% were Native Hawaiian or Pacific Islander. There were 13.8% Hispanic or Latino participants.
The primary immunogenicity objectives were to assess CYFENDUS vaccine-induced TNA 50% neutralization factor (NF50) response at Day 64 and non-inferiority to BioThrax following two doses of CYFENDUS administered intramuscularly two weeks apart (Week 0 and 2) and three doses of BioThrax administered subcutaneously two weeks apart (Week 0, 2, and 4). The two co-primary immunogenicity endpoints and statistical evaluation criteria were:
- •
- Percentage of CYFENDUS recipients achieving a threshold TNA NF50 value ≥0.56 at Day 64 (with the lower bound of the 2-sided 95% confidence interval [CI] for the proportion ≥40%) and,
- •
- The percent difference between participants achieving a threshold TNA NF50 value ≥0.29 at Day 64 in those who received CYFENDUS and those who received BioThrax (with the lower bound of the 2-sided 95% CI for the difference being greater than -15%).
The TNA NF50 thresholds of 0.56 and 0.29 were based on the TNA NF50 values in rabbits and nonhuman primates, respectively, that were associated with 70% probability of survival in pre-exposure prophylaxis studies, in which animals were immunized with BioThrax and subsequently challenged with aerosolized B. anthracis spores [see Animal Toxicology and/or Pharmacology (13.2)].
Overall, 66.3% of CYFENDUS participants from the PP population achieved threshold TNA NF50 value ≥0.56 on Day 64 in the study; the lower bound of the 95% CI was 64.4% (Table 2).
Table 2. Percentage of Participants Achieving TNA NF50 Threshold in Clinical Study 4 at Day 64 After Administration of Two Doses of CYFENDUS (Per Protocol Population) Day 64 CYFENDUS CI = Confidence interval; NF50 = 50% neutralization factor; PP = Per protocol; TNA = Toxin neutralizing antibody. CYFENDUS was considered to have achieved a protective level of immunity at Day 64 if the lower bound for the two-sided 95% CI for the percentage of participants with TNA NF50 values above the specified threshold of protection (≥0.56) was ≥40%. N= number of participants in PP population
2543
Percent (95% CI) of participants meeting the TNA NF50 ≥0.56 threshold
66.3 (64.4, 68.1)
The percent of participants with a threshold TNA NF50 of ≥0.29 at Day 64 was 86.6% in the CYFENDUS group compared with 61.4% in the BioThrax group, corresponding to a difference of 25.2%. The lower bound of the 2-sided 95% CI for the difference was 20.5%, thereby demonstrating non-inferiority of CYFENDUS to BioThrax (Table 3).
Table 3. Non-inferiority of CYFENDUS to BioThrax Based on Difference in Percentage of Participants Achieving TNA NF50 Threshold in Clinical Study 4 at Day 64 (Per Protocol Population) CI = Confidence interval; N = Number of participants in the study arm in the Per Protocol population; % = Percent of participants achieving the target TNA NF50 threshold; NF50 = 50% neutralization factor; TNA = Toxin-neutralizing antibody Non-inferiority to BioThrax is based on the lower bound of the two-sided 95% CI for the difference in percentage (CYFENDUS – BioThrax) being above -15%. CYFENDUS
(N=2543)%
[95% CI]
BioThrax
(N=430)%
[95% CI]
Difference (CYFENDUS-BioThrax) %
[95% CI]
Percent of Participants with TNA NF50 ≥0.29
86.6
[85.2, 87.9]
61.4
[56.6, 66.0]
25.2
[20.5, 30.1]
14.2 Concomitant Administration with Ciprofloxacin or Doxycycline
A randomized, open-label, multicenter study (Study 3; NCT04067011) was conducted in males and females, age 18 to 45 years, to investigate the potential interactions of concomitant intramuscular administration of CYFENDUS with oral administration of ciprofloxacin or doxycycline. The potential effect of CYFENDUS vaccination on ciprofloxacin or doxycycline serum levels was assessed by evaluating steady-state pharmacokinetic profiles of ciprofloxacin or doxycycline before and after vaccination with a two-dose series of CYFENDUS.
Concomitant administration of 0.5 mL of CYFENDUS intramuscularly with oral ciprofloxacin or doxycycline in participants did not have a clinically relevant impact on the pharmacokinetics of ciprofloxacin and doxycycline, or the immunogenicity of CYFENDUS as measured by the TNA assay.
- 15 REFERENCES
-
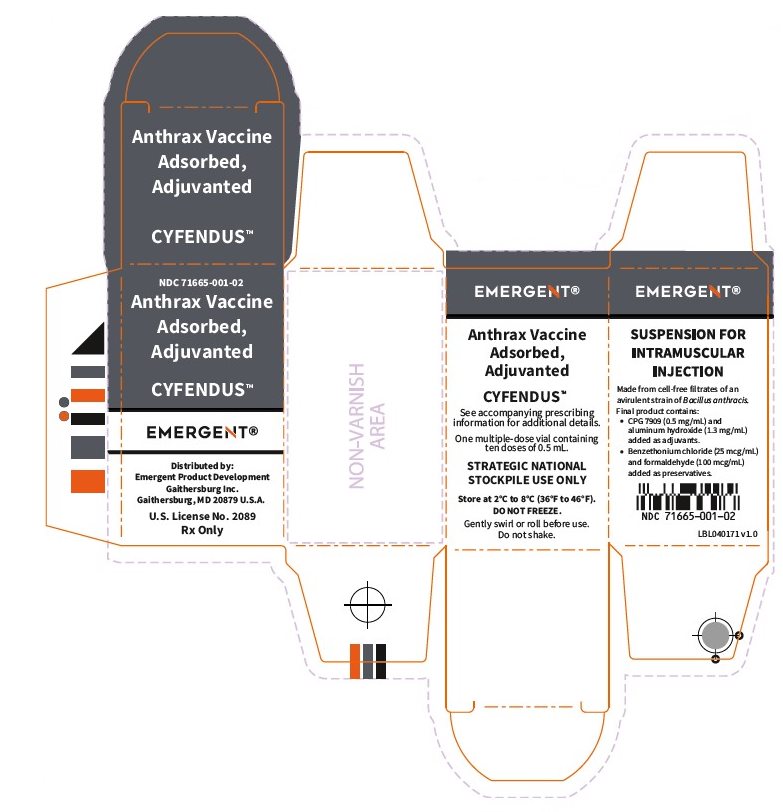
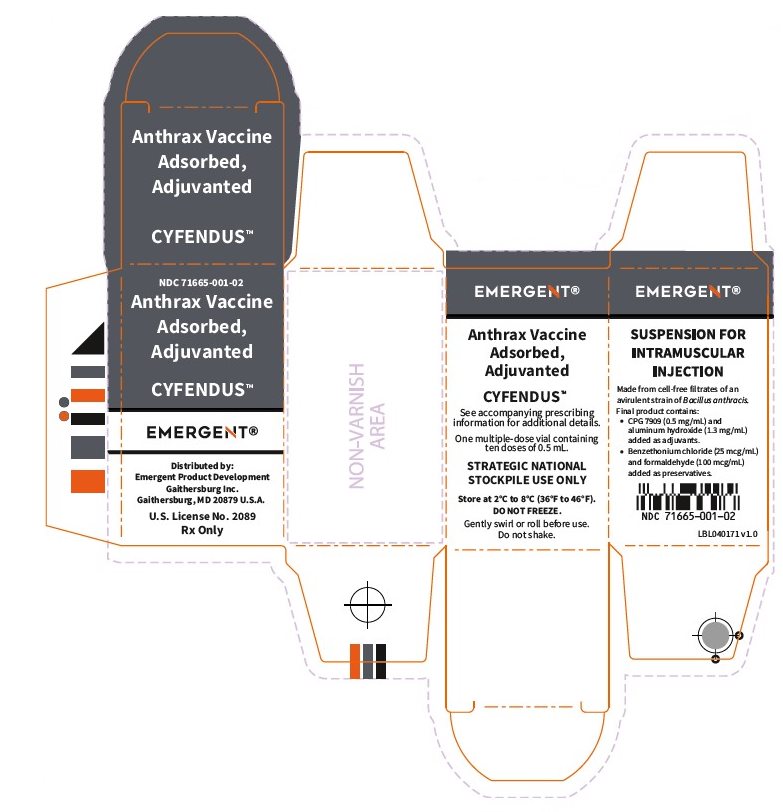
16 HOW SUPPLIED/STORAGE AND HANDLING
CYFENDUS is supplied in multiple-dose vials containing ten 0.5 mL doses.
NDC 71665-001-01 (vial), 71665-001-02 (carton)
Store at 2°C to 8°C (36°F to 46°F).
Protect vials from light.
To avoid foaming DO NOT shake.
Do not freeze. Discard if product has been frozen.
Do not use CYFENDUS after the expiration date printed on the label.
The vial stoppers are not made with natural rubber latex.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advise women of the potential risk to the fetus.
Inform patients of the benefits and risks of immunization with CYFENDUS.
Instruct patients to report any serious adverse reaction to their healthcare provider.
Manufactured by:
Emergent BioDefense Operations Lansing LLC
Lansing, MI USA 48906
License No. 1755Distributed by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, MD USA 20879
License No. 2089CYFENDUS™, BioThrax® and any and all Emergent BioSolutions Inc. brands, products, services and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries.
-
PATIENT PACKAGE INSERT
Patient Information
CYFENDUS™ (Anthrax Vaccine Adsorbed, Adjuvanted) Suspension for Intramuscular Injection
Please read this Patient Information summary carefully before you get this vaccine. This summary does not take the place of talking with your healthcare provider about CYFENDUS. If you have questions or would like more information, please talk with your healthcare provider.
What is CYFENDUS?
- •
- CYFENDUS is a vaccine licensed by the FDA to protect against anthrax disease when given with recommended antibacterial drugs in persons 18 through 65 years of age after exposure to anthrax.
- •
- CYFENDUS may not protect all vaccine recipients from anthrax disease.
- •
- How well CYFENDUS works when given after exposure to anthrax has been studied only in animals. It has not been studied in humans because there are not enough people who get the disease naturally, and it is not ethical to expose people to anthrax on purpose to find out how well CYFENDUS works.
- •
- The safety of CYFENDUS was studied in adults 18 through 65 years of age.
Who should not get CYFENDUS?
You should not get CYFENDUS if you have a history of severe allergic reaction to any ingredient of the vaccine, including aluminum hydroxide, benzethonium chloride, formaldehyde, or CPG 7909 or had a serious reaction after getting CYFENDUS or BioThrax® (Anthrax Vaccine Adsorbed) previously
What should I tell my healthcare provider before getting CYFENDUS?
- •
- If you may be pregnant, plan to get pregnant soon, or are nursing a baby.
- •
- About medicines that you take, including over-the-counter medicines and supplements.
- •
- About immune problems you have, including steroid treatments and cancer treatments.
What if I discover I was pregnant at the time I got CYFENDUS?
- •
- Inform your healthcare provider
How is CYFENDUS given?
CYFENDUS is given as an injection in your muscle.
After getting the first dose, you should come back for the second dose two weeks later. It is important that you get both doses.
You are being given CYFENDUS because you may have been exposed to anthrax. It is important that you also take antibacterial drugs as recommended by your healthcare provider.
What are the possible or reasonably likely side effects of CYFENDUS?
The most common side effects of CYFENDUS are:
- •
- Pain/tenderness, warmth, hardness, itching, swelling, redness, and bruising at the injection site
- •
- Problems moving the arm in which you received the vaccine
- •
- Muscle aches
- •
- Tiredness
- •
- Headache
- •
- Fever
Tell your healthcare provider about any side effects that concern you and ask about acceptable treatments for relief of side effects. There may be other side effects that are not listed here. Talk to your healthcare provider if you have questions or concerns about possible side effects.
You may report side effects to FDA by calling 1-800-822-7967 or to the website www.vaers.hhs.gov. You may also report side effects directly to Emergent BioSolutions at 1-800-768-2304 or at medicalinformation@ebsi.com.
What are the ingredients in CYFENDUS?
CYFENDUS does not contain live bacteria. CYFENDUS contains bacterial proteins that are not alive and do not cause anthrax disease. CYFENDUS also contains aluminum hydroxide and CPG 7909 as adjuvants (substances added to some vaccines to enhance the immune response of vaccinated individuals), and benzethonium chloride and formaldehyde as preservatives.
Distributed by:
Emergent Product Development Gaithersburg Inc.Gaithersburg, MD USA 20879
License No. 2089
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 07/2023
-
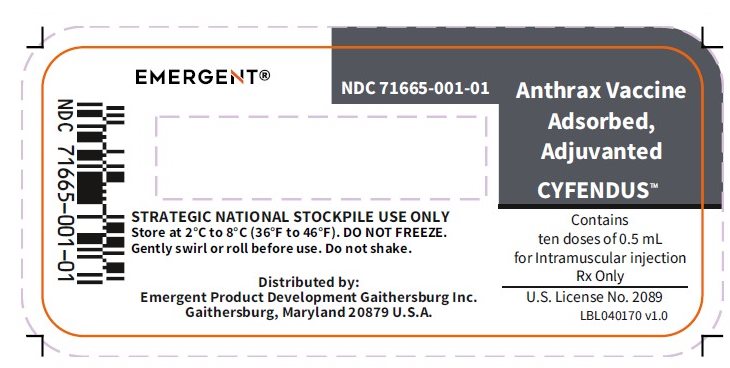
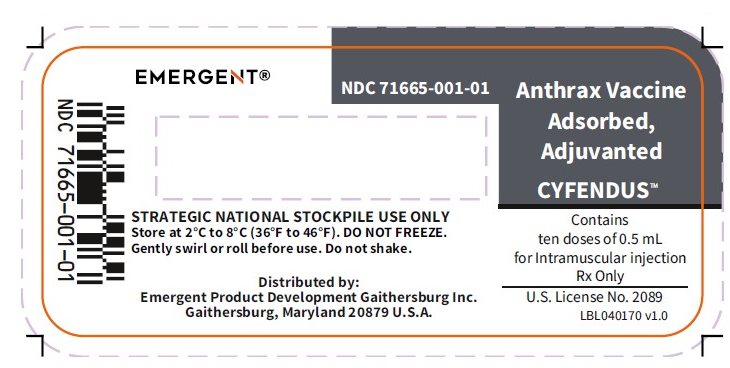
PRINCIPAL DISPLAY PANEL
NDC 71665-001-01
Anthrax Vaccine Adsorbed, Adjuvanted
CYFENDUS™
Contains
ten doses of 0.5 mL
for Intramuscular injection
Rx Only
U.S. License No. 2089
LBL040170 v1.0
STRATEGIC NATIONAL STOCKPILE USE ONLY
Store at 2°C to 8°C (36°F to 46°F). DO NOT FREEZE.
Gently swirl or roll before use. Do not shake.
Distributed by:
Emergent Product Development Gaithersburg Inc.
Gaithersburg, Maryland 20879 U.S.A.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CYFENDUS
anthrax vaccine adsorbed, adjuvanted injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:71665-001 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACILLUS ANTHRACIS STRAIN V770-NP1-R ANTIGENS (UNII: 873OI62848) (BACILLUS ANTHRACIS STRAIN V770-NP1-R ANTIGENS - UNII:873OI62848) BACILLUS ANTHRACIS STRAIN V770-NP1-R ANTIGENS 100 ug in 1 mL Inactive Ingredients Ingredient Name Strength AGATOLIMOD SODIUM (UNII: I4Z5C8FM6H) 0.5 mg in 1 mL ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) 1.3 mg in 1 mL BENZETHONIUM CHLORIDE (UNII: PH41D05744) 25 ug in 1 mL FORMALDEHYDE (UNII: 1HG84L3525) 100 ug in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.5 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71665-001-02 10 in 1 CARTON 1 NDC:71665-001-01 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125761 09/19/2023 Labeler - Emergent Product Development Gaithersburg Inc. (189488554) Registrant - Emergent Product Development Gaithersburg Inc. (189488554) Establishment Name Address ID/FEI Business Operations Emergent BioDefense Operations Lansing LLC 026489018 MANUFACTURE(71665-001)