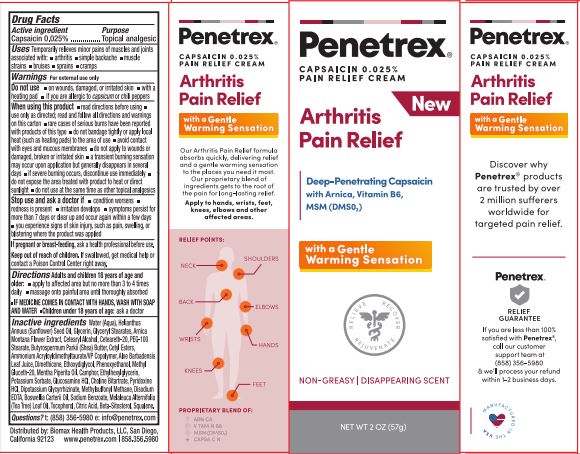
Label: PENETREX(R) ARTHRITIS PAIN RELIEF- capsaicin cream
- NDC Code(s): 62742-4216-1, 62742-4216-2
- Packager: Allure Labs
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
-
WHEN USING
When using this product
Read directions befbre using
use only as directed; read and follow all directions and warnings on this carton rare cases of serious bums have been reported with products of this type
do not bandage tightly or apply local heat (such as heating pads) to the area of use
avoid contact with eyes and mucous membranes
do not apply to wounds or damaged, broken or irritated skin
a transient burning sensation may occur upon application but generally disappears in several days
if severe burning occurs, discontinue use immediately
do not expose the area treated With product to heat or direct sunlight
do not use at the same time as other topical analgesics,
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients:
Water (Aqua),Helanthus Annuus (Sunflower) Seed Oil, Glycerin,GlycerylStearates, Amica Montana Flower Extract,
Cetearyl Alcohol,Ceteareth-20,PEG-100 Stearate, Butyrospermum Parkii (Shea) Buffer,Cetyl Esters,Ammonium Acryloyldimethyltaurate/VP Copolymer, Aloe Barbadensis Leaf Juice,Dimethicone, Ethoxydiglycol, Phenoxyethanol,
Methyl Gluceth-20,Menthe Piperita Oil,Camphor,Ethylhexylglycerin,Potassium Sorbate, Glucosamine HCI,Choline Bitartrate,
Pyridoxine HCL, Glycyrrhizinate Dipotassium, Methyl sulfonyl Methane,Disodium EDTA, Boswellia carterii oil,
Sodium Benzoate, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Tocopherol, Citric Acid,Beta-Sitosterol, Squalene.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PENETREX(R) ARTHRITIS PAIN RELIEF
capsaicin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62742-4216 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.025 g in 100 g Inactive Ingredients Ingredient Name Strength TEA TREE OIL (UNII: VIF565UC2G) AMMONIUM ACRYLOYLDIMETHYLTAURATE (UNII: KBC00G95HI) ALOE VERA LEAF (UNII: ZY81Z83H0X) CAMPHOR (NATURAL) (UNII: N20HL7Q941) FRANKINCENSE OIL (UNII: 67ZYA5T02K) TOCOPHEROL (UNII: R0ZB2556P8) SUNFLOWER OIL (UNII: 3W1JG795YI) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-100 STEARATE (UNII: YD01N1999R) SHEA BUTTER (UNII: K49155WL9Y) PEPPERMINT OIL (UNII: AV092KU4JH) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) DIMETHICONE (UNII: 92RU3N3Y1O) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) CHOLINE BITARTRATE (UNII: 6K2W7T9V6Y) .BETA.-SITOSTEROL (UNII: S347WMO6M4) SQUALENE (UNII: 7QWM220FJH) CETYL ESTERS WAX (UNII: D072FFP9GU) METHYL GLUCETH-20 (UNII: J3QD0LD11P) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62742-4216-2 1 in 1 CARTON 06/20/2022 1 NDC:62742-4216-1 57 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/20/2022 Labeler - Allure Labs (926831603) Registrant - Allure Labs (926831603) Establishment Name Address ID/FEI Business Operations Allure Labs 926831603 manufacture(62742-4216)