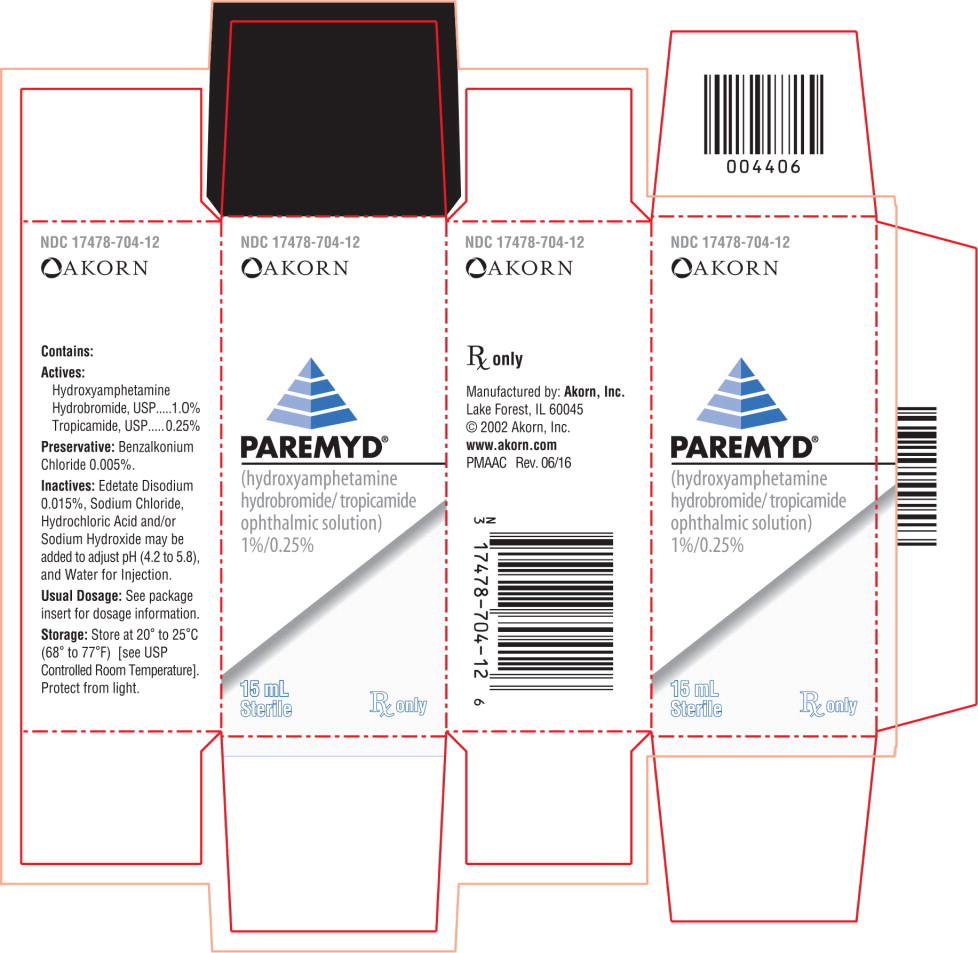
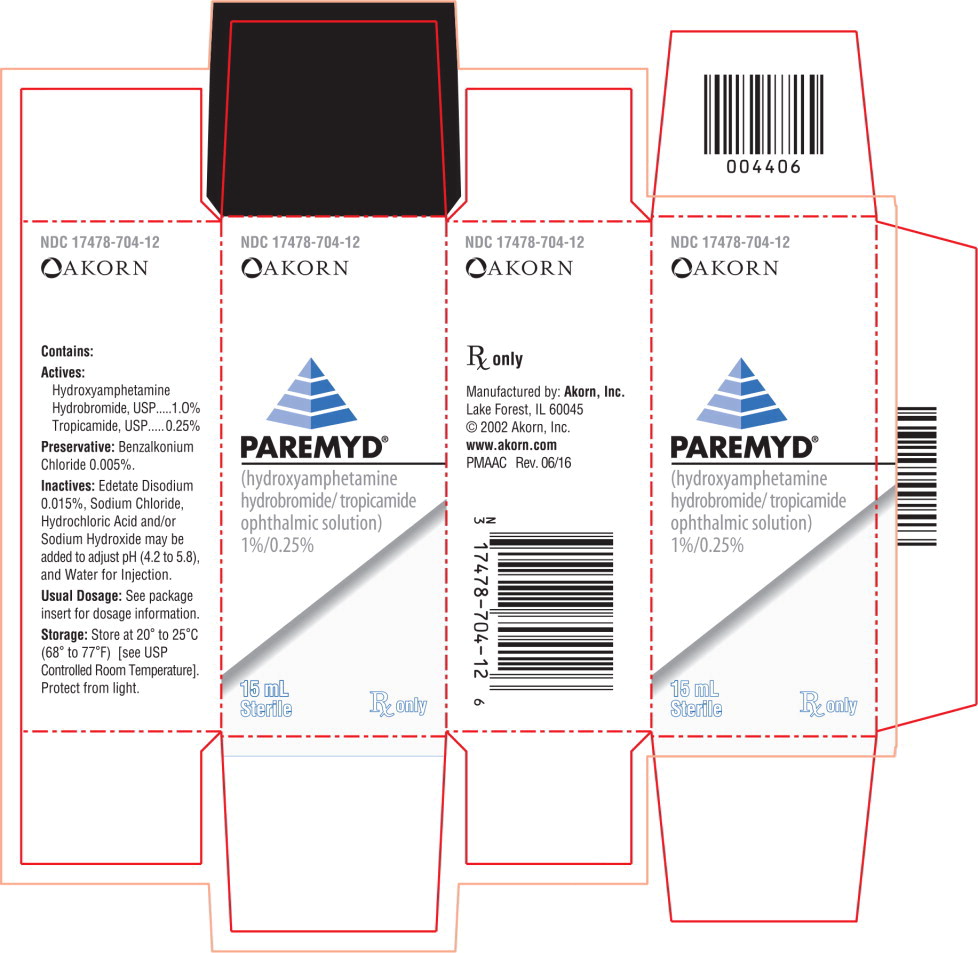
Label: PAREMYD- hydroxyamphetamine hydrobromide, tropicamide solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 17478-704-12 - Packager: Akorn
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
PAREMYD® sterile ophthalmic solution is a topical mydriatic combination product for ophthalmic use.
STRUCTURAL FORMULAE

CHEMICAL NAME
Hydroxyamphetamine hydrobromide: Phenol, 4-(2-aminopropyl)-, hydrobromide
Tropicamide: Benzeneacetamide, N-ethyl-α-(hydroxymethyl)-N-(4-pyridinylmethyl)-CONTAINS
Actives: Hydroxyamphetamine hydrobromide, USP.......................1.0%
Tropicamide, USP.................................................................0.25%
Preservative: Benzalkonium Chloride 0.005%
Inactives: Edetate Disodium 0.015%, Sodium Chloride; Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (4.2 to 5.8 during its shelf life), and Water for Injecton. The osmolality of PAREMYD® is approximately 307 mOsm/l. -
CLINICAL PHARMACOLOGY
PAREMYD® Solution combines the effects of the adrenergic agent, hydroxyamphetamine hydrobromide, and the anticholinergic agent, tropicamide.
Hydroxyamphetamine hydrobromide is an indirectly-acting sympathomimetic agent which, when applied topically to the eye, causes the release of endogenous norepinephrine from intact adrenergic nerve terminals resulting in mydriasis. Since hydroxyamphetamine hydrobromide has little or no direct activity on the receptor site, dilation does not usually occur if there is damage to the presynaptic nerve terminal, e.g., Horner's Syndrome. However, it is not known whether damage to the presynaptic nerve terminal will influence the extent of mydriasis produced by PAREMYD®. Hydroxyamphetamine hydrobromide has minimal cycloplegic action.
Tropicamide is a parasympatholytic agent which, when applied topically to the eye, blocks the responses of the sphincter muscle of the iris and the ciliary muscle to cholinergic stimulation, producing dilation of the pupil and paralysis of the ciliary muscle. Tropicamide produces short-duration mydriasis. Although cycloplegia occurs with higher doses of tropicamide, there is evidence with 0.25% tropicamide that full cycloplegia does not occur.
Since both these agents act on different effector sites, their simultaneous use produces an additive mydriatic effect. PAREMYD® provides diminished pupil responsiveness to light, facilitating ophthalmoscopy. The onset of action with PAREMYD® occurs within 15 minutes, followed by maximum effect within 60 minutes after instillation of one drop. Clinically significant dilation, inhibition of pupillary light response, and partial cycloplegia last 3 hours, with recovery beginning at approximately 90 minutes and with complete recovery occurring in most patients in 6 to 8 hours. However, in some cases complete recovery may take up to 24 hours. Effectiveness may differ slightly in patients with light and dark irides, with those patients with light irides experiencing a slightly greater mydriasis.
- INDICATIONS
- CONTRAINDICATIONS
-
WARNINGS
For topical ophthalmic use only; not for injection.
There is evidence that mydriatics may produce a transient elevation of intraocular pressure in patients with open-angle glaucoma.
This preparation rarely may cause CNS disturbances which may be particularly dangerous in infants, children or the aged. Psychotic reactions, behavioral disturbances and vasomotor or cardio-respiratory collapse have been reported with the use of anticholinergic drugs.
-
PRECAUTIONS
General
Patients with hypertension, hyperthyroidism, diabetes or cardiac disease (i.e., arrhythmias or chronic ischemic heart disease) should be monitored after instillation. The elderly and others in whom glaucoma or increased intraocular pressure may be encountered following administration of PAREMYD® Solution should also be monitored closely. To avoid inducing angle-closure glaucoma, an estimation of the depth of the angle of the anterior chamber should be made.
Information for Patients
Patients should be advised not to touch the dropper tip to any surface since this may contaminate the solution. Patients should be advised to use caution when driving or engaging in other hazardous activities while pupils are dilated. Patients may experience photophobia and/or blurred vision and should protect their eyes in bright illumination when pupils are dilated. Parents should be warned not to get this preparation in their child's mouth and to wash their own hands and the child's hands following administration.
Carcinogenesis, mutagenesis, impairment of fertility
No studies have been performed to evaluate the carcinogenic, mutagenic or impairment of fertility potential of PAREMYD®.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when PAREMYD® is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. PAREMYD® may rarely cause CNS disturbances which may be dangerous in infants and children. Psychotic reactions, behavioral disturbances and vasomotor or cardio-respiratory collapse in children have been reported with the use of anticholinergic drugs. (See WARNINGS). Keep this and all medications out of the reach of children.
-
ADVERSE REACTIONS
Increased intraocular pressure has been reported following use of mydriatics. Transient stinging, dryness of the mouth, blurred vision, photophobia with or without corneal staining, tachycardia, headache, allergic reactions, nausea, vomiting, pallor and muscle rigidity have been reported with the use of tropicamide and/or hydroxyamphetamine hydrobromide, and thus may occur with PAREMYD® Solution. Central nervous system disturbances have also been reported. Psychotic reactions, behavioral disturbances, and vasomotor or cardiorespiratory collapse have been reported with the use of anticholinergic drugs.
Rare but serious cardiovascular events, including death due to myocardial infarction, ventricular fibrillation and significant hypotensive episodes have occurred shortly following PAREMYD® instillation.
-
OVERDOSAGE
Ocular overdosage will cause dilation of the pupils. Systemic overdosage or ingestion of large doses may result in hypertension, cardiac arrhythmias, sub-sternal discomfort, headache, sweating, nausea, vomiting and gastrointestinal irritation. Patients with systemic overdosage should be carefully monitored and treated symptomatically.
-
DOSAGE AND ADMINISTRATION
One to two drops in the conjunctival sac. The onset of action with PAREMYD® Solution occurs within 15 minutes followed by maximum effect within 60 minutes. Clinically significant dilation, inhibition of pupillary light response, and partial cycloplegia last 3 hours.
Mydriasis will reverse spontaneously with time, typically in 6 to 8 hours. However, in some cases, complete recovery may take up to 24 hours.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAREMYD
hydroxyamphetamine hydrobromide, tropicamide solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17478-704 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hydroxyamphetamine hydrobromide (UNII: 59IG47SZ0E) (Hydroxyamphetamine - UNII:FQR280JW2N) Hydroxyamphetamine hydrobromide 10 mg in 1 mL Tropicamide (UNII: N0A3Z5XTC6) (Tropicamide - UNII:N0A3Z5XTC6) Tropicamide 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Edetate Disodium (UNII: 7FLD91C86K) Sodium Chloride (UNII: 451W47IQ8X) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Benzalkonium Chloride (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17478-704-12 1 in 1 CARTON 01/30/1992 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019261 01/30/1992 Labeler - Akorn (117693100) Establishment Name Address ID/FEI Business Operations Akorn 117696840 MANUFACTURE(17478-704) , ANALYSIS(17478-704) , STERILIZE(17478-704) , PACK(17478-704) , LABEL(17478-704)