Label: TISSUEBLUE- brilliant blue g injection, solution
- NDC Code(s): 68803-722-05
- Packager: D.O.R.C. Dutch Ophthalmic Research Center (International) B.V.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
TissueBlue: These highlights do not include all the information needed to use TissueBlue 0.025% safely and effectively. See full prescribing information for TissueBlue 0.025%. Initial U.S. Approval: 2019
INDICATIONS AND USAGE
TissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025% is is a disclosing agent indicated to selectively stain the internal limiting membrane (ILM). (1)
DOSAGE AND ADMINISTRATION
- Inject TissueBlue 0.025% directly in a Balanced Salt Solution (BSS)-filled vitreous cavity.
- Excess TissueBlue should be removed from the vitreous cavity.
DOSAGE FORMS AND STRENGTHS
TissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025% is supplied in 2.25 mL syringes filled to a volume of 0.5 mL. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
Excessive staining: Excess TissueBlue 0.025% should be removed from the eye immediately after staining.
Use of the syringe: Make sure the plunger moves smoothly before injecting the solution. (5)ADVERSE REACTIONS
Adverse reactions that have been reported in procedures that included the use of TissueBlue 0.025% have often been associated with the surgical procedure. The complications include retinal (retinal break, tear, hemorrhage, and detachment and cataracts. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Dutch Ophthalnic, USA at 1-800-75-DUTCH or FDA at
1-800-FDA-1088 or www.fda.gov/medwatch (6)Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
TissueBlue 0.025% - Indications & Usage Section
TissueBlue 0.025% - Dosage & Administration Section
TissueBlue 0.025% - Dosage forms & Strengths section
TissueBlue 0.025% - Contraindications section
TissueBlue 0.025% - Warnings and Precautions section
TissueBlue 0.025% - Adverse Reactions section
TissueBlue 0.025% - Use in specific populations section
TissueBlue 0.025% - Pregnancy section
TissueBlue 0.025% - Lactation section
TissueBlue 0.025% - Pediatric use section
TissueBlue 0.025% - Geriatric use section
TissueBlue 0.025% - Description section
TissueBlue 0.025% - Clinical Pharmacology section
TissueBlue 0.025% - Mechanism of action section
TissueBlue 0.025% - Nonclinical toxicology section
TissueBlue 0.025% - Nonclinical toxicology section
TissueBlue 0.025% - How supplied section
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- TissueBlue 0.025% - Indications & Usage Section
-
TissueBlue 0.025% - Dosage & Administration Section
TissueBlue 0.025% is carefully injected into the Balanced Salt Solution (BSS)-filled vitreous cavity using a blunt cannula attached to the pre-filled syringe, without allowing the cannula to contact the retina or allowing TissueBlue to get under the retina. Sufficient staining is expected within a few seconds. Following staining, all excess dye should be removed from the vitreous cavity.
- TissueBlue 0.025% - Dosage forms & Strengths section
- TissueBlue 0.025% - Contraindications section
- TissueBlue 0.025% - Warnings and Precautions section
- TissueBlue 0.025% - Adverse Reactions section
-
TissueBlue 0.025% - Use in specific populations section
TissueBlue 0.025% - Pregnancy section
Risk Summary
There are no available data on the use of TissueBlue 0.025% in pregnant women to inform a drug associated risk. Systemic absorption of TissueBlue 0.025% in humans is expected to be negligible following intravitreal injection and subsequent removal of the drug at the completion of surgical procedures. Due to the negligible systemic exposure, it is not expected that maternal use of TissueBlue 0.025% will result in fetal exposure to the drug.
Adequate animal reproduction studies were not conducted with TissueBlue 0.025%.TissueBlue 0.025% - Lactation section
Risk Summary
No data are available regarding the presence of Brilliant Blue G in human milk after intraocular administration of TissueBlue 0.025%, or the effects on the breastfed infant or the effects on milk production. However, breastfeeding is not expected to result in exposure of the child to Brilliant Blue G due to the expected negligible systemic exposure of BBG in humans following intravitreal injection and subsequent removal of the drug at the completion of surgical procedures. -
TissueBlue 0.025% - Description section
TissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025% is a sterile solution of BBG (a dye). Each mL of TissueBlue 0.025% contains BBG 0.25 mg, Polyethylene Glycol 40mg and Buffered Sodium Chloride solution (8.20 mg of sodium chloride, 3.10 mg sodium phosphate dibasic dodecahydrate, 0.30 mg sodium phosphate monobasic dihydrate, water for injection). The pH range of TissueBlue 0.025% Solution is between 7.3 and 7.6.
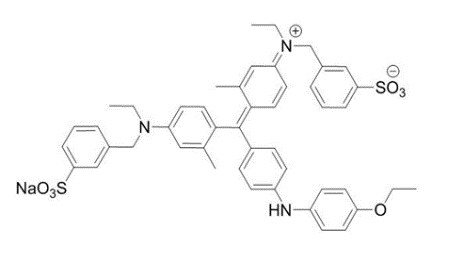
The drug substance BBG has the chemical name Brilliant Blue G, a molecular weight of 854.02 and has the following chemical structure:Molecular formula: C 47H 48N 3NaO 7S 2
- TissueBlue 0.025% - Clinical Pharmacology section
-
TissueBlue 0.025% - Nonclinical toxicology section
TissueBlue 0.025% - Nonclinical toxicology section
Studies to evaluate the potential for carcinogenicity or impairment of fertility of TissueBlue 0.025% have not been conducted.
Brilliant Blue G was not mutagenic in the Ames assay, the in vitro mouse lymphoma assay, or the in vivo rat micronucleus assay. -
TissueBlue 0.025% - How supplied section
TissueBlue (Brilliant Blue G Ophthalmic Solution), 0.025% is supplied as 0.5 mL of Brilliant Blue G Ophthalmic Solution, 0.025% in a sterile, single-dose Luer Lok, 2.25 mL glass syringe, with a grey rubber plunger stopper and tip cap with polypropylene plunger rod in a pre-formed polypropylene blister pouch sealed with a Tyvek® lid.
NDC 68803-722-05 (One 0.5 mL syringe)
NDC 68803-722-25 (Carton of five 0.5 mL syringes) - TissueBlue 0.025% - Storage and Handling section
- SPL UNCLASSIFIED SECTION
-
Package Label - 0.5 mL
TissueBlue
(Brilliant Blue G Ophthalmic Solution) 0.025%
Staining Solution for Ophthalmic Surgery
Protect from light, frost and moisture. Store at 15°C to 25°C (59°F to 77°F). Sterile.
Active ingredients/Ingrédient actif: Brilliant Blue G 0.025% Inactive ingredients/Ingrédients inactifs: Water for injection, Sodium chloride, Sodium phosphate dibasic dihydrate, Sodium phosphate monobasic dihydrate, Polyethylene Glycol.
NDC 68803-722-05 (One 0.5 mL syringe)
NDC 68803-722-25 (Carton of five 0.5 mL syringes)
-
INGREDIENTS AND APPEARANCE
TISSUEBLUE
brilliant blue g injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68803-722 Route of Administration INTRAOCULAR, OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRILLIANT BLUE G (UNII: M1ZRX790SI) (BRILLIANT BLUE G - UNII:M1ZRX790SI) BRILLIANT BLUE G 0.0125 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.2 mg in 0.5 mL SODIUM PHOSPHATE, DIBASIC, DODECAHYDRATE (UNII: E1W4N241FO) 3.1 mg in 0.5 mL SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) 0.3 mg in 0.5 mL WATER (UNII: 059QF0KO0R) 4743 g in 0.5 mL POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) 2 mg in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68803-722-05 10 in 1 CARTON 12/31/2019 1 1 in 1 POUCH 1 0.5 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209569 12/31/2019 Labeler - D.O.R.C. Dutch Ophthalmic Research Center (International) B.V. (407522184) Registrant - D.O.R.C. Dutch Ophthalmic Research Center (International) B.V. (407522184) Establishment Name Address ID/FEI Business Operations Pharmpur GmbH 340805167 manufacture(68803-722)