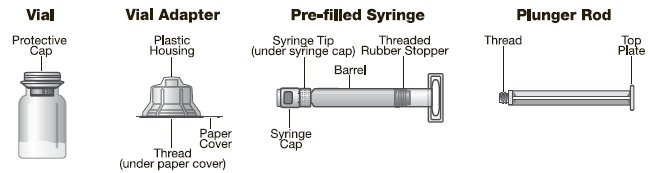
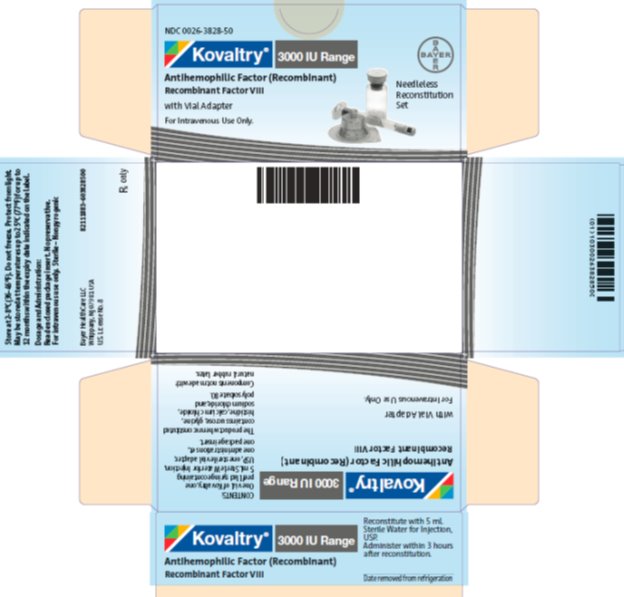
Label: KOVALTRY (antihemophilic factor- recombinant kit
-
NDC Code(s):
0026-0426-02,
0026-0426-05,
0026-0426-15,
0026-3821-25, view more0026-3821-99, 0026-3822-25, 0026-3822-99, 0026-3824-25, 0026-3824-99, 0026-3826-50, 0026-3826-99, 0026-3828-50, 0026-3828-99, 0026-4821-01, 0026-4821-99, 0026-4822-01, 0026-4822-99, 0026-4824-01, 0026-4824-99, 0026-4826-01, 0026-4826-99, 0026-4828-01, 0026-4828-99
- Packager: Bayer HealthCare LLC
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KOVALTRY safely and effectively. See full prescribing information for KOVALTRY.
KOVALTRY [Antihemophilic Factor (Recombinant)]
Lyophilized Powder for Solution for Intravenous Injection – Reconstitution with Vial Adapter
Initial U.S. Approval: 2016INDICATIONS AND USAGE
KOVALTRY®, Antihemophilic Factor (Recombinant), is a recombinant, human DNA sequence derived, full length Factor VIII concentrate indicated for use in adults and children with hemophilia A (congenital Factor VIII deficiency) for:
• On-demand treatment and control of bleeding episodes
• Perioperative management of bleeding
• Routine prophylaxis to reduce the frequency of bleeding episodes
KOVALTRY is not indicated for the treatment of von Willebrand disease (1).
DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
Control of bleeding episodes and perioperative management (2.1)
- •
- Required dose (IU) = body weight (kg) x desired Factor VIII rise (% of normal or IU/dL) x reciprocal of expected/observed recovery (e.g., 0.5 for a recovery of 2 IU/dL per IU/kg).
- •
- Estimated Increment of Factor VIII (IU/dL or % of normal) = [Total Dose (IU)/body weight (kg)] x 2 (IU/dL per IU/kg).
Routine prophylaxis (2.1)
- •
- Adults and adolescents: 20-40 IU/kg 2 or 3 times per week.
- •
- Children ≤12 years old: 25-50 IU/kg 2 times per week, 3 times per week or every other day.
DOSAGE FORMS AND STRENGTHS
- KOVALTRY is available as lyophilized powder in single-dose vials containing nominally 250, 500, 1000, 2000, or 3000 IU. Each vial of KOVALTRY contains the labeled amount of recombinant Factor VIII in IU (3).
CONTRAINDICATIONS
Do not use in patients who have history of hypersensitivity reactions to the active substance, mouse or hamster protein, or other constituents of the product (4).
WARNINGS AND PRECAUTIONS
- •
- Hypersensitivity reactions, including anaphylaxis, are possible. Should symptoms occur, discontinue treatment with KOVALTRY and administer appropriate treatment (5.1).
- •
- Development of Factor VIII neutralizing antibodies has occurred. Perform an assay that measures Factor VIII inhibitor concentration if expected plasma Factor VIII activity levels are not attained, or if bleeding is not controlled as expected with administered dose (5.2, 5.5).
ADVERSE REACTIONS
The most frequently reported adverse reactions in clinical trials (≥5%) were inhibitors in previously untreated patients (PUPs)/minimally treated patients (MTPs), pyrexia, headache, and rash (6).
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Pediatric Use: Due to higher clearance (body weight adjusted) in children ≤12 years of age, higher or more frequent dosing may be needed (8.4).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation and Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Neutralizing Antibodies
5.3 Cardiovascular Risk Factors
5.4 Catheter-related Infections
5.5 Monitoring Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
- KOVALTRY, Antihemophilic Factor (Recombinant), is a recombinant, human DNA sequence derived, full length Factor VIII concentrate indicated for use in adults and children with hemophilia A (congenital Factor VIII deficiency) for:
- •
- On-demand treatment and control of bleeding episodes
- •
- Perioperative management of bleeding
- •
- Routine prophylaxis to reduce the frequency of bleeding episodes
KOVALTRY is not indicated for the treatment of von Willebrand disease.
-
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
2.1 Dose
- •
- Dosage and duration of treatment depend on the severity of the Factor VIII deficiency, the location and extent of bleeding, and the patient’s clinical condition. Careful control of replacement therapy is especially important in cases of major surgery or life-threatening bleeding episodes.
- •
- Each vial label of KOVALTRY states the Factor VIII potency in international units (IU). One IU is defined by the current WHO (World Health Organization) international standard (IS) for Factor VIII concentrate.
- •
- Potency assignment for KOVALTRY is determined using a chromogenic substrate assay. A field study involving 41 clinical laboratories from around the world measured recoveries of KOVALTRY spiked into hemophilic plasma. The results of the field study indicated that the Factor VIII activity of KOVALTRY can be accurately measured in plasma using either a one-stage clotting or chromogenic substrate assay according to routine methods of the testing laboratory.
- •
- The required dose for a desired Factor VIII level expressed as IU/dL (or % of normal) can be estimated using the following formula:
-
Required dose (IU) = body weight (kg) x desired Factor VIII rise (% of normal or IU/dL)
x reciprocal of expected/observed recovery (e.g., 0.5 for a recovery of 2 IU/dL per IU/kg) - The expected in vivo peak increase of Factor VIII level expressed as IU/dL (or % of normal) can be estimated using the following formula:
-
Estimated increment of Factor VIII (IU/dL or % of normal) = [Total dose (IU)/body weight (kg)]
x 2 (IU/dL per IU/kg) - Examples (assuming patient’s baseline Factor VIII is <1%):
- •
- A peak of 50% [50 IU/dL] is required in a 20 kg child. In this situation, the required dose of KOVALTRY would be 20 kg x 50 IU/dL x 0.5 (for recovery of 2 IU/dL per IU/kg) = 500 IU
- •
- A dose of 2000 IU of KOVALTRY administered to a 50 kg patient should be expected to result in post-infusion Factor VIII increase of 2000 IU / 50 kg (body weight) x 2 IU/dL per IU/kg = 80 IU/dL (80% of normal)
- •
- Adjust dose to the patient’s clinical response. Patients may vary in their pharmacokinetic (e.g., half-life, incremental recovery) and clinical responses to KOVALTRY.
- •
- On-demand Treatment and Control of Bleeding Episodes
A guide for dosing KOVALTRY for the on-demand treatment and control of bleeding episodes is provided in Table 1. The goal of treatment is to maintain a plasma Factor VIII activity level at or above the plasma levels (in % of normal or in IU/dL) outlined in Table 1.
Table 1: Dosing for Control of Bleeding Episodes Degree of Bleeding
Factor VIII Level Required
(IU/dL or % of normal)Frequency of Doses (hours)
Duration of Therapy (days)
Minor (Early hemarthrosis, minor muscle, oral bleeds)
20–40
Repeat every
12–24 hoursAt least 1 day, until bleeding episode as indicated by pain is resolved or healing is achieved
Moderate (More extensive hemarthrosis, muscle bleeding, or hematoma)
30–60
Repeat every
12–24 hours3 to 4 days or more until pain and acute disability are resolved
Major (intracranial, intra-abdominal or intrathoracic hemorrhages, gastrointestinal bleeding, central nervous system bleeding, bleeding in the retropharyngeal or retroperitoneal spaces, or iliopsoas sheath, life or limb threatening hemorrhage)
60–100
Repeat every
8–24 hoursUntil bleeding is resolved
Perioperative Management of Bleeding
A guide for dosing KOVALTRY during surgery (perioperative management) is provided in Table 2. The goal of treatment is to maintain a plasma Factor VIII activity level at or above the plasma level (in % of normal or in IU/dL) outlined in Table 2. During major surgery, monitoring with appropriate laboratory tests, including serial Factor VIII activity assays, is highly recommended [see Warnings and Precautions (5.5)].
Table 2: Dosing for Perioperative Management Type of Surgery
Factor VIII Level Required
(IU/dL or % of normal)Frequency of Doses (hours)
Duration of Therapy (days)
Minor (Such as tooth extraction)
30–60
(pre- and post-operative)Repeat every 24 hours
At least 1 day until healing is achieved
Major (Such as intracranial, intra-abdominal, intrathoracic, or joint replacement surgery)
80–100
(pre- and post-operative)Repeat every
8–24 hoursUntil adequate wound healing is complete, then continue therapy for at least another 7 days to maintain Factor VIII activity of
30–60% (IU/dL)Routine Prophylaxis
- •
- Individualize the patient’s dose based on clinical response.
- •
- Adults and adolescents: 20 to 40 IU of KOVALTRY per kg of body weight two or three times per week.
- •
- Children ≤12 years old: 25 to 50 IU of KOVALTRY per kg body weight twice weekly, three times weekly, or every other day according to individual requirements [see Use in Specific Populations (8.4)].
2.2 Preparation and Reconstitution
- •
- Reconstitute and administer KOVALTRY with the components provided with each package. If any component of the package is opened or damaged, do not use this component.
The procedures below are provided as general guidelines for the reconstitution of KOVALTRY using the sterile vial adapter with a 15 micrometer filter and a prefilled diluent syringe, which together serve as an alternative needleless reconstitution system.
Usability Testing of Vial Adapter
Usability testing was conducted with 60 users, including 15 pediatric hemophilia A patients (between 10-17 years of age), 15 adult hemophilia A patients (≥18 years of age), 15 caregivers, and 15 healthcare providers. To mimic real life, the pediatric and adult patients and the caregivers were given minimal training, which included participants performing a supervised reconstitution and later performing a single unaided reconstitution. Healthcare providers were untrained in this study and could learn the procedure from the provided Instructions for Use. All participants were able to successfully and safely use the vial adapter device for reconstitution.
Reconstitution
- •
- Work on a clean surface and wash hands thoroughly using soap and warm water before performing the procedures.
- •
- Reconstitute KOVALTRY with the components provided with each package. If any component of the package is opened or damaged, do not use this component.
- •
- Filter the reconstituted product to remove potential particulate matter in the solution. Filtering is achieved by using the vial adapter.
Pooling
If the dose requires more than one vial, reconstitute each vial as described above with the diluent syringe provided. Use a larger plastic syringe (not provided) to combine the content of the vials into the syringe.
2.3 Administration
For intravenous use only.
- •
- Inspect reconstituted KOVALTRY visually for particulate matter and discoloration prior to administration. Do not use if you notice any particulate matter or discoloration.
- •
- Administer reconstituted KOVALTRY as soon as possible. If not, store at room temperature for no longer than 3 hours.
- •
- Infuse KOVALTRY intravenously over a period of 1 to 15 minutes. Adapt the rate of administration to the response of each individual patient.
-
3 DOSAGE FORMS AND STRENGTHS
KOVALTRY is available as a lyophilized powder in single-dose glass vials containing nominally 250, 500, 1000, 2000, or 3000 IU of recombinant Factor VIII per vial.
Each vial of KOVALTRY is labeled with actual Factor VIII potency expressed in IU determined using a chromogenic substrate assay. This potency assignment employs a Factor VIII concentrate standard that is referenced to the current WHO International Standard for Factor VIII concentrate, and is evaluated by appropriate methodology to ensure accuracy of the results.
-
4 CONTRAINDICATIONS
KOVALTRY is contraindicated in patients who have a history of hypersensitivity reactions to the active substance, to any of the excipients, or to mouse or hamster proteins [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, are possible with KOVALTRY. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include chest or throat tightness, dizziness, mild hypotension and nausea. Discontinue KOVALTRY if symptoms occur and seek immediate emergency treatment.
KOVALTRY may contain trace amounts of mouse and hamster proteins [see Description (11)]. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
5.2 Neutralizing Antibodies
Neutralizing antibody (inhibitor) formation has occurred following administration of KOVALTRY. Previously untreated patients (PUPs) are at greatest risk for inhibitor development with all Factor VIII products [see Adverse Reactions (6.1)]. Carefully monitor patients for the development of Factor VIII inhibitors, using appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled as expected with administered dose, suspect the presence of an inhibitor (neutralizing antibody) [see Warnings and Precautions (5.5)].
5.3 Cardiovascular Risk Factors
Hemophilic patients with cardiovascular risk factors or diseases may be at the same risk to develop cardiovascular events as non-hemophilic patients when clotting has been normalized by treatment with Factor VIII.
5.4 Catheter-related Infections
Catheter-related infections may be observed when KOVALTRY is administered via central venous access devices (CVADs). These infections have not been associated with the product itself.
5.5 Monitoring Laboratory Tests
- •
- Monitor plasma Factor VIII activity levels using a validated test to confirm that adequate Factor VIII levels have been achieved and maintained [see Dosage and Administration (2.1)].
- •
- Monitor for development of Factor VIII inhibitors. Perform a Bethesda inhibitor assay if expected Factor VIII plasma levels are not attained or if bleeding is not controlled with the expected dose of KOVALTRY. Use Bethesda Units (BU) to report inhibitor titers.
-
6 ADVERSE REACTIONS
The most frequently reported adverse reactions in clinical trials (≥5%) were inhibitors in previously untreated patients (PUPs)/minimally treated patients (MTPs), pyrexia, headache, and rash (see Table 3).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety profile of KOVALTRY was evaluated in 236 (193 previously treated (PTPs), 43 PUPs/MTPs) patients, inclusive of 94 pediatric patients <12 years of age. The safety analysis was done using a pooled database from three multi-center, prospective, open-label clinical studies. The median time on clinical trial for the pooled safety population was 558 days with a median of 183 exposure days (EDs). The majority of patients (N=201) accumulated ≥ 100 EDs.
The total number of exposure days to KOVALTRY for all treatments including peri-operative management was 65029 EDs. Subjects who received KOVALTRY for perioperative management (N=5) with treatment period of 2 to 3 weeks and those who received single doses of KOVALTRY for PK studies (N=6) were excluded from the pooled safety analysis. Table 3 lists the adverse reactions reported during clinical studies. For PTPs, the frequency, type, and severity of adverse reactions in children are similar to those in adults.
Table 3: Adverse Reactions from pooled clinical studies (all age groups): Clinical trials 1, 2, and 3) (N=236) MedDRA Primary System Organ Class
Preferred termFrequency of Adverse Drug Reactions
N (%)Blood and the Lymphatic System Disorders
Lymphadenopathy
2 (0.8%)
Cardiac Disorders
Palpitation
Sinus tachycardia
2 (0.8%)
2 (0.8%)
Gastrointestinal Disorders
Abdominal pain
Abdominal discomfort
Dyspepsia
9 (3.8%)
3 (1.3%)
4 (1.7%)
General Disorders and Administration Site Conditions
Pyrexia
Chest discomfort
Injection site reactions*
22 (9.3%)
2 (0.8%)
6 (2.5%)
Immune System Disorders
Hypersensitivity
1 (0.4%)
Nervous System Disorders
Dizziness
Dysgeusia
Headache
3 (1.3%)
1 (0.4%)
20 (8.5%)
Psychiatric Disorders
Insomnia
5 (2.1%)
Skin and Subcutaneous Tissue Disorders
Dermatitis allergic
Pruritus
Rash†
Urticaria
2 (0.8%)
6 (2.5%)
13 (5.5%)
3 (1.3%)
Vascular disorders
Flushing
1 (0.4%)
In PTPs (N=193), Factor VIII inhibitors occurred in 1 patient at a frequency of 0.5% (1/193) and in PUPs/MTPs (N=43) with a frequency of 54.8% (23/42); inhibitor analysis was based on 42 of 43 PUPs/MTPs, as one patient was unevaluable for inhibitor analysis and thus excluded.
Immunogenicity
All clinical trial subjects were monitored for neutralizing antibodies (inhibitors) to Factor VIII by the modified Bethesda assay using blood samples obtained prior to the first infusion of KOVALTRY, at defined intervals during the studies and at the completion visit.
Clinical trials with KOVALTRY evaluated a total of 204 treated pediatric and adult patients diagnosed with severe hemophilia A (Factor VIII <1%) with previous exposure to Factor VIII concentrates ≥50 EDs, no PTP developed neutralizing antibodies to Factor VIII. One case of transient low titer inhibitor (0.6 BU/mL (peak titer: 1.0 BU/mL)) occurred in a 13 year old PTP after 549 EDs concurrent with an acute infection and positive IgG anticardiolipin antibodies. The Factor VIII recovery was normal (2.7 IU/dL per IU/kg), annualized bleeding rate (ABR) was zero, and no change in therapy was required.
In the main phase of the clinical trial in PUPs/MTPs, Factor VIII inhibitors were detected in 23 of 42 patients (54.8%, 95% CI: 39 to 70%) with a median (range) of 9 (4-42) EDs at the time of the first positive inhibitor test. Of these, 6 (14.3%) patients had low titer inhibitors (≤ 5.0 BU) who continued on treatment and had negative inhibitor results at the end of the study. In 17 (40.5%) patients who developed high titer inhibitors (> 5.0 BU), high-risk mutations for inhibitor development were identified in 12 (85.7%) of 14 patients with available FVIII mutation data.
The detection of antibody formation is dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, it may be misleading to compare the incidence of antibodies to KOVALTRY with the incidence of antibodies to other products.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with KOVALTRY use in pregnant women to inform on drug-associated risk. Animal reproduction studies have not been conducted using KOVALTRY. It is not known whether KOVALTRY can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of KOVALTRY in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for KOVALTRY and any potential adverse effects on the breastfed infant from KOVALTRY or from the underlying maternal condition.
8.4 Pediatric Use
Safety and efficacy studies with KOVALTRY have been performed in 51 pediatric PTPs ≤12 years of age and 43 pediatric PUPs/MTPs <6 years of age [see Clinical Studies (14)].
Body weight adjusted clearance of Factor VIII in children ≤12 years of age is higher than in adults and adolescents. Consider higher or more frequent dosing in children to account for this difference in clearance [see Clinical Pharmacology (12.3)].
8.5 Geriatric Use
Clinical studies with KOVALTRY did not include patients aged 65 and over to determine whether or not they respond differently from younger patients. However, clinical experience with other Factor VIII products has not identified differences between the elderly and younger patients. As with any patient receiving recombinant Factor VIII, dose selection for an elderly patient should be individualized.
-
11 DESCRIPTION
KOVALTRY, Antihemophilic Factor (Recombinant), is a sterile, non-pyrogenic, white to slightly yellow powder for reconstitution contained in a single-dose vial. The final product does not contain any preservative. The reconstituted product is indicated for intravenous administration. The product is available in 250 IU, 500 IU, 1000 IU, 2000 IU, or 3000 IU nominal potencies; however, for each dosage strength the actual, assayed Factor VIII potency is directly printed on each vial label. The container closure system consists of a 10 mL, Type I glass vial sealed with a bromobutyl grey stopper and an aluminum crimp seal with plastic flip-off cap plus vial adapter. The vial adapter was designed to connect with the sterile water for injection (sWFI), prefilled diluent syringe. KOVALTRY is formulated with the following excipients: 2.2% glycine, 1% sucrose, 30 mM sodium chloride, 2.5 mM calcium chloride, 20 mM histidine and 80 ppm polysorbate 80. The pH of the reconstituted product is 6.6 to 7.0. Intravenous administration of sucrose contained in KOVALTRY will not affect blood glucose level.
The active substance in KOVALTRY is the unmodified full length recombinant Factor VIII glycoprotein comprising the human derived amino acid sequence. Post-translational modifications are similar to those of endogenous Factor VIII including glycosylation sites and sulfation of tyrosine sites. Manufacturing and quality controls ensure that both galactose-alpha-1,3-galactose (alpha-Gal) and N-glycolyl neuraminic acid (NGNA) content are below the 1% limit of detection established for each analytical method.
KOVALTRY is produced by a genetically engineered Baby Hamster Kidney (BHK) cell line into which the human Factor VIII gene was introduced together with the human heat shock protein 70 (HSP 70) gene. HSP 70 is an intracellular protein that improves proper folding of the Factor VIII protein. While KOVALTRY and Kogenate FS have the same protein backbone, human- and animal-derived raw materials are not added to the cell culture, purification, or formulation processes for KOVALTRY. In the manufacturing process for KOVALTRY, recombinant Factor VIII is secreted into cell culture medium and is purified from process- and product-related impurities using a series of chromatography and filtration steps. The production process incorporates two dedicated viral clearance steps: (1) a detergent treatment step for inactivation and (2) a 20 nanometer filtration step for removal of viruses and potential protein aggregates.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
KOVALTRY temporarily replaces the missing clotting Factor VIII that is needed for effective hemostasis.
12.2 Pharmacodynamics
Plasma clotting time as measured by the activated partial thromboplastin time (aPTT) is prolonged in patients with hemophilia A. Treatment with KOVALTRY normalizes the aPTT.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of KOVALTRY were investigated in PTPs (0 to 61 years of age) with severe Hemophilia A following administration of 50 IU/kg of KOVALTRY. The PK parameters of KOVALTRY are presented in Table 4 (one-stage clotting assay) and Table 5 (chromogenic substrate assay). The PK of KOVALTRY were similar between single and repeat dosing (in 19 subjects following 6 to 12 months of prophylaxis).
Table 4: Pharmacokinetic Parameters [Arithmetic Mean ± SD] for KOVALTRY (50 IU/kg dose), One-Stage Clotting Assay Parameter [unit]
12 to 17 yrs (N=5)
≥18 yrs
(N=21)AUC [IU*h/dL]
1013.9 ± 286.8
1601.3 ± 520.0
Cmax [IU/dL]
91.7 ± 28.7
99.7 ± 14.9
t½ [h]
11.7 ± 1.11
14.3 ± 3.7
MRTIV [h]
16.1 ± 0.8
19.8 ± 5.7
Vss [dL/kg]
0.85 ± 0.24
0.63 ± 0.11
CL [dL/h/kg]
0.053 ± 0.017
0.035 ± 0.012
AUC: area under the curve
Cmax: maximum drug concentration in plasma after single dose
t½ : terminal half-life
MRTIV: mean residence time after an IV administration
Vss: apparent volume distribution at steady-state
CL: clearance
The PK parameters of KOVALTRY for subjects across age groups are shown in Table 5. In general, children <12 years of age demonstrated lower plasma concentrations when compared to PTP children ≥12 years of age.
Table 5: Pharmacokinetic Parameters [Arithmetic Mean ± SD] for KOVALTRY (50 IU/kg dose), Chromogenic Substrate Assay Parameter [unit]
0 to <2 yrs
(N=4)
2 to <6 yrs
(N=6)
6 to <12 yrs
(N=10)b12 to 17 yrs
(N=5)≥18 yrs
(N=21)AUC [IU*h/dL]
1232.5 ± 581.3
1484.8 ± 411.3a
1214.5 ± 395.1
1572.0 ± 448.0
2103.4 ± 702.8
Cmax [IU/dL]
96.1 ± 20.4
83.3 ± 28.7
81.6± 17.8
132.5 ± 46.3
133.1 ± 20.4
t½ [h]
9.6 ± 3.1
12.2 ± 3.1a
12.0 ± 2.1
14.4 ± 5.5
14.2 ± 3.5
MRTIV [h]
14.1 ± 4.7
17.9 ± 4.1a
17.8 ± 2.9
19.8 ± 5.8
19.9 ± 4.9
Vss [dL/kg]
0.63 ± 0.18
0.60 ± 0.15a
0.79 ± 0.23
0.71 ± 0.39
0.50 ± 0.11
CL [dL/h/kg]
0.050 ± 0.024
0.034 ± 0.011a
0.045 ± 0.016
0.034 ± 0.010
0.027 ± 0.010
a n=5
b One subject considered PK outlier was excludedIncremental Recovery analysis after 6 months of prophylactic treatment yielded comparable results with incremental recovery after the first dose (see Table 6).
Table 6: Incremental Recovery in PTPs 0 to <6 yrs
N=256 to 12 yrs
N=25≥12 yrs
N=115Chromogenic substrate assay resultsa
Median (Q1; Q3)
(IU/dL per IU/kg)
1.6 (1.3; 1.9)
1.7 (1.4; 2.0)
2.3 (1.8; 2.6)
One-stage assay resultsa
Median (Q1; Q3)
(IU/dL per IU/kg)
-
-
2.2 (1.8; 2.4)
aStart of trial
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of KOVALTRY or other studies to determine the effects of KOVALTRY on fertility have not been performed. KOVALTRY was negative in the modified in-vitro (Mammalian Mutation and Chromosome Aberration Assay with Mouse Lymphoma Cells) genotoxicity test. KOVALTRY is expected to have no mutagenic potential.
-
14 CLINICAL STUDIES
The safety and efficacy of KOVALTRY for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis in subjects with severe hemophilia A (<1% Factor VIII) was evaluated in three international (including U.S.) clinical studies. Immunocompetent subjects with severe hemophilia A (Factor VIII activity ≤1%) and no history of Factor VIII inhibitors were eligible for the trials.
Trial 1: a multi-center, open-label, cross-over, uncontrolled, study in adolescent and adult (age ≥12 years to <65 years) PTPs (≥150 EDs) evaluated the pharmacokinetics, efficacy and safety of routine prophylaxis, and perioperative management of bleeding of KOVALTRY (see Table 7). The primary efficacy variable was ABR. The prophylactic regimen was 20 to 50 IU/kg two or three times per week in which the dosing frequency was assigned by the investigator based on the subject’s individual requirements.
Trial 2: a multi-center, open-label, cross-over, uncontrolled, randomized study in adolescent and adult (age ≥12 years to <65 years) PTPs (≥150 EDs) evaluated the superiority of prophylaxis over on-demand treatment with KOVALTRY over a one-year treatment period (see Table 7). The primary efficacy variable was ABR. The prophylactic regimen was 20 to 30 IU/kg two times per week or 30 to 40 IU/kg three times per week and the treatment group was assigned by randomization.
Trial 3: a multi-center, open-label, uncontrolled study that evaluated the pharmacokinetics, efficacy and safety of routine prophylaxis, and perioperative management of bleeding of KOVALTRY (see Table 8) in previously treated patients (PTPs; ≥50 EDs) ≤12 years of age (Part A) and previously untreated patients (PUPs) and minimally treated patients (MTPs; ≤3 EDs) <6 years of age (Part B). The primary efficacy variable was annualized number of total bleeds during routine prophylaxis that occurred within 48 hours following previous prophylaxis infusion. ABR during prophylaxis, independent of time of infusion, was also analyzed. In Part A, the prophylactic regimen was 25 to 50 IU/kg at frequencies of either 2 times per week, 3 times per week or every other day and could be adapted to an individual subject’s need by the investigator.
In all studies, treatments of breakthrough bleeds and perioperative management were at the investigator’s discretion based on standard of care.
A total of 204 previously treated subjects (153 subjects ≥12 years of age and 51 subjects <12 years of age) and 43 PUPs/MTPs <6 years of age were treated in the clinical trials. Amongst the PTPs, 174 subjects were treated for at least 12 months and 78 of these subjects were treated for 24 months. Amongst the PUPs/MTPs, 34 subjects were treated for at least 12 months and 20 of these subjects were treated for 24 months.
Table 7: Overview of Trial 1 (Prophylaxis Treatment Phase) and Trial 2 Trial 1
(N=62)Trial 2
(N=80)Age: mean ± SD
31.5 ± 12.7 years
29.6 ± 11.0 years
Previous treatment: %
Prophylaxis: 80.6%
On-demand: 100%
Number of Target joints at baseline:
mean ± SD1.4 ± 1.3
3.0 ± 2.1
Joint hemorrhage history
(during 12 months prior to study):
mean ± SD of joint bleeds8.0 ± 11.9
32.1 ± 23.8
Table 8: Overview of Trial 3 Trial 3 PTPs 0 to <6 yrs
(N=25)PTPs 6 to 12 yrs
(N=26)Age: mean ± SD (range)
3.8 ± 1.3 years (1-5)
8.8 ± 1.8 years (6-11)
Previous treatment: %
Prophylaxis: 92.0%
Prophylaxis: 65.4%
Number of Target joints at baseline: mean ± SD
0.2 ± 0.4
0.7 ± 1.1
On-demand Treatment and Control of Bleeding Episodes
Adolescents and Adults
A total of 1892 bleeding episodes in 110 subjects were treated with KOVALTRY in Trial 1 and Trial 2 (see Table 9). The majority of the bleeding episodes were spontaneous, localized in joints, and mild to moderate in severity.
In Trial 1 and Trial 2, the treatment responses in a total of 1859 treated bleeds were assessed by the subjects compared to their previous treatment experience.
Table 9: On-demand Treatment and Control of Bleeding Episodes in Adolescents and Adults Treated with KOVALTRY Characteristics of Bleeding Episodes
Trial 1
Trial 2
Prophylaxis
Main Study
N=62Prophylaxis
Extension
N=55Prophylaxis
N=59On-demand
N=21Total number of bleeds
241
154
293
1204
Spontaneous: n/total (%)
153/241 (63.5%)
79/150* (52.7%)
209/283* (73.9%)
943/1202* (78.5%)
Trauma: n/total (%)
79/241 (32.8%)
70/150* (46.7%)
74/283* (26.1%)
258/1202* (21.5%)
Joint bleeds: n/total (%)
191/241 (79.3%)
120/154 (77.9%)
255/293 (87.0%)
924/1197* (77.2%)
Mild/moderate: n/total (%)
215/241 (89.2%)
130/153* (84.9%)
260/293 (88.8%)
1092/1196* (91.3%)
% of bleeds treated with ≤2 infusions
87.0%
96.2%
95.3%
Response to treatment of bleeds assessed as “Excellent” or “Good”: n/total† (%)
190/235 (80.9%)
107/149 (71.8%)
172/279 (61.6%)
834/1196 (69.7%)
Median dose per infusion (range)
31.6 IU/kg
(14-67 IU/kg)29.4 IU/kg
(19-49 IU/kg)22.0 IU/kg
(11-35 IU/kg)Children 12 Years of Age and Younger
A total of 97 bleeding episodes in 28 previously treated pediatric subjects and 105 bleeding episodes in 37 PUPs/MTPs were treated with KOVALTRY. Majority (96.9% in PTPs and 97.1% in PUPs/MTPs) of the bleeds were mild to moderate in severity for both groups. Fifty-nine (72.8%) and 62 (59.0%) bleeds were trauma related for the previously treated subjects and PUPS/MTPs, respectively. During the 6 month treatment period, the median dose of KOVALTRY for the treatment of breakthrough bleeds in the previously treated subjects was 36.94 IU/kg per infusion (range 20.8–71.6 IU/kg).
Assessment of response to treatment of bleeds was as follows:
Excellent: Abrupt pain relief and/or improvement in signs of bleeding with no additional infusion administered; Good: Definite pain relief and/or improvement in signs of bleeding but possibly requiring more than one infusion for complete resolution; Moderate: Probable or slight improvement in signs of bleeding with at least one additional infusion for complete resolution; Poor: No improvement at all between infusions or condition worsens.
The hemostatic efficacy in on-demand treatment of bleeds was assessed as either “good” or “excellent” in 90.1% of cases (97.8% in the younger age group and 81.0% in the older age group). Majority of bleeds (89.7%) were successfully treated with ≤2 infusions. Response to treatment was similar for children aged 0 to <6 compared to 6 to 12 years of age (see Table 10).
Table 10: On-demand Treatment and Control of Bleeding Episodes in Children Treated with KOVALTRY Characteristics of Bleeding Episodes Trial 3 PTPs 0 to <6 yrs
(N=25)PTPs 6 to 12 yrs
(N=26)PTPs 0 to 12 yrs
(N=51)Total number of bleeds
52
45
97
Spontaneous: n/total (%)
8/44* (18.2%)
12/37* (32.4%)
20/81* (24.7%)
Trauma: n/total (%)
36/44* (81.8%)
23/37* (62.2%)
59/81* (72.8%)
Joint bleeds: n/total (%)
10/52 (19.2%)
22/45 (48.9%)
32/97 (33.0%)
Mild/moderate: n/total (%)
50/52 (96.2%)
44/45 (97.8%)
94/97 (96.9%)
% of bleeds treated with ≤2 infusions
92.4%
86.7%
89.7%
Response to treatment of bleeds assessed as “Excellent” or “Good”: n/total† (%)
43/44 (97.8%)
30/37 (81.0%)
73/81 (90.1%)
Median dose per infusion (range)
38.7 IU/kg
(20.8–71.6 IU/kg)32.4 IU/kg
(21.7–50.0 IU/kg)36.9 IU/kg
(20.8–71.6 IU/kg)Perioperative Management
A total of 14 major and 46 minor surgeries were performed in 44 previously treated subjects (43 adults and adolescents and 1 child under 12 years of age) with severe hemophilia A. Seven of the 14 major surgeries in PTPs were orthopedic procedures, including joint replacement. Approximately 51% of the minor surgeries in PTPs were dental extractions. All subjects received KOVALTRY as bolus infusions. In the adolescent and adult subjects, the initial KOVALTRY doses administered ranged between 3000–5000 IU. The median total dose on the day of surgery was 107.5 IU/kg (range 60–207 IU/kg). In a single previously treated subject younger than 12 years of age who underwent a major surgery, the total initial KOVALTRY dose administered was 2500 IU (108.7 IU/kg).
The blood loss, during and after surgery, was within expected ranges. Hemostatic control was assessed by surgeons as “good” (perioperative bleeding slightly but not clinically significantly increased over expectations for the non-hemophilic patient; treatment similar to non-hemophilic patient) or “excellent” (perioperative blood loss similar to the non-hemophilic patient).
Routine Prophylaxis
Adolescents and Adults
A total of 140 subjects were treated with KOVALTRY for at least 12 months with median (range) 157 EDs (25-178) in Trial 1, [305 EDs (25-355) inclusive of extension phase], and 153 EDs (103-187) in Trial 2 (see Table 11). In both studies, subjects in the Intent-to-Treat (ITT) population received 95% to 100% of the prescribed number of prophylaxis infusions.
Table 11: Prophylaxis Treatment with KOVALTRY in Adolescents and Adults – Treatment Exposure Trial 1
(N=62)*Trial 2
(N=59)- *
- Trial 1 included PK, safety and efficacy of prophylaxis and hemostasis during surgeries. Prophylaxis phase data are presented.
Median nominal prophylaxis dose/ infusion (range)
All
Prophylaxis 2 times per week
Prophylaxis 3 times per week
31.2 IU/kg (21-43 IU/kg)
35.0 IU/kg (21-42 IU/kg)
31.1 IU/kg (24-43 IU/kg)
31.7 IU/kg (21-42 IU/kg)
30.4 IU/kg (21-34 IU/kg)
37.4 IU/kg (30-42 IU/kg)
Treatment duration
1 year main study
1 year
Trial 1: 2 times per week (n=18); 3 times per week (n=44)
Trial 2: 2 times per week (n=28); 3 times per week (n=31)
The mean and median ABR for the ITT population in Trial 1 was 3.8 ± 5.2 and 1 bleed/year, respectively. In Trial 2, comparison of the bleeding rates between subjects receiving on-demand therapy versus prophylaxis in an ANOVA demonstrated a statistically significant difference (p<0.0001) in the median ABR in subjects receiving on-demand therapy (60 bleeds per year) as compared to subjects receiving prophylaxis (2 bleeds per year). In Trial 2, mean ABR in subjects receiving on-demand therapy was 57.7 ± 24.6 versus 4.9 ± 6.8 in the subjects receiving prophylaxis.
Table 12: ABR in Adolescent and Adult Subjects - *
- IQR = Interquartile Range
- †
- Month 1-6 refers to the first six months of the treatment period and Month 7-12 refer to the second six months of the treatment period
- ‡
- Observation of one-year treatment period
- §
- n=total number of subjects with zero bleeds
- ¶
- n=total number of subjects randomized to treatment arms
Trial 1
(N=62)Trial 2
(N=59)2 times per week
(n=18)3 times per week
(n=44)2 times per week
(n=28)3 times per week
(n=31)ABR Median (IQR*Q1; Q3)
- All Bleeds
1.0 (0.0; 8.0)
2.0 (0.5; 5.0)
- Spontaneous Bleeds
0.5 (0.0; 2.0)
1.0 (0.0; 3.9)
2.0 (0.0; 6.5)
0.0 (0.0; 3.0)
- Joint Bleeds
0.5 (0.0; 7.0)
1.8 (0.0; 3.0)
2.5 (0.0; 7.5)
1.0 (0.0; 4.0)
Subjects with Zero Bleeding Episodes‡ % (n)
37.5% (6/16§)
62.5% (10/16§)
28.6% (8/28¶)
25.8% (8/31¶)
The ABR for subjects (n=21) receiving on-demand therapy in Trial 2 [median (IQR Q1; Q3)] for all bleeds: 60 (41.7; 76.3); spontaneous bleeds: 42.1 (24.3; 61.3); joint bleeds: 38.8 (24.3; 60.0).
Children 12 Years of Age and Younger
In Part A, a total of 51 PTPs were treated with KOVALTRY for at least 6 months with median (range) 73 EDs (37-103) (see Table 13). Subjects received >95% of the prescribed number of prophylaxis infusions.
Table 13: Prophylaxis Treatment with KOVALTRY in Children 12 Years of Age or Younger – Treatment Exposure Trial 3 PTPs 0 to <6 yrs
(N=25)PTPs 6 to 12 yrs
(N=26)- *
- Treatment regimen at the start of the study. Study duration was six months.
Treatment regimen* during study (6 months) n (%)
2 times per week
3 times per week or every other day
9 (36%)
16 (64%)
13 (50%)
13 (50%)
Nominal prophylaxis dose per infusion, median (range)
36.4 IU/kg (21-58 IU/kg)
31.8 IU/kg (22-50 IU/kg)
In children 12 years of age and younger (n=51), the median (IQR Q1; Q3) ABR within 48 hours after prophylactic infusion was 0 (0; 4) for all bleeds, and 0 (0; 0) for spontaneous and joint bleeds. The median (IQR Q1; Q3) ABR during prophylactic treatment independent of time of infusion was 1.9 (0; 6) for all bleeds, 0 (0; 0) for spontaneous bleeds and 0 (0; 2) for joint bleeds. The mean ABR within 48 hours after prophylactic infusion was 2.04 ± 2.91. The mean ABR at any time during the prophylaxis regimen was 3.75 ± 4.98.
In both age groups (0 to <6 years and 6 to 12 years), the ABR for spontaneous bleeds and joint bleeds within 48 hours after prophylactic treatment [ABR median (IQR Q1; Q3)] was 0 (0; 0). The median (IQR Q1; Q3) annualized number of spontaneous bleeds during prophylactic treatment independent of time of infusion was 0 (0; 0). The median (IQR Q1; Q3) annualized number of joint bleeds during prophylactic treatment independent of time of infusion was 0 (0; 1.9) in 0 to <6 years age group and 0 (0; 2.1) in 6 to 12 years age group (see Table 14).
The majority (32/53) of bleeds that occurred within 48 hours after a previous prophylaxis infusion were trauma related. Twenty-three (45.1%) subjects reported no bleeds during the six-month prophylaxis period.
Table 14: ABR in Children 12 Years of Age or Younger Trial 3 PTPs 0 to <6 yrs
(N=25)PTPs 6 to 12 yrs
(N=26)Within 48 hrs after prophylactic treatment
During prophylactic treatment*
Within 48 hrs after prophylactic treatment
During prophylactic treatment*
All Bleeds ABR Median (IQR†Q1; Q3)
1.9 (0.0; 4.0)
2.0 (0.0; 6.0)
0.0 (0.0; 2.0)
0.9 (0.0; 5.8)
Number of Subjects with Zero Bleeding Episodes (%)
10 (40%)
13 (50%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
KOVALTRY is available as a lyophilized powder in single-dose glass vials, one vial per carton. It is supplied with a sterile vial adapter with 15-micrometer filter and a prefilled diluent glass barrel syringe, which together serve as a needleless reconstitution system. The prefilled diluent syringe contains Sterile Water for Injection, USP. An administration set is also provided in the package. Available sizes:
Nominal Strength (IU)
Diluent (mL)
Kit NDC Number
Color Code
250
2.5
0026-3821-25
Blue
500
2.5
0026-3822-25
Green
1000
2.5
0026-3824-25
Red
2000
5.0
0026-3826-50
Yellow
3000
5.0
0026-3828-50
Gray
Actual Factor VIII activity in IU is stated on the label of each KOVALTRY vial.
The product vial and diluent syringe are not made with natural rubber latex.
Storage and Handling
Product as Packaged for Sale
- •
- Store KOVALTRY at +2°C to +8°C (36°F to 46°F) for up to 30 months from the date of manufacture. Do not freeze. Within this period, KOVALTRY may be stored for a single period of up to 12 months at temperatures up to +25°C or 77°F.
- •
- Record the starting date of room temperature storage on the unopened product carton. Once stored at room temperature, do not return the product to the refrigerator. The shelf-life then expires after storage at room temperature for 12 months, or after the expiration date on the product vial, whichever is earlier.
- •
- Do not use KOVALTRY after the expiration date indicated on the vial.
- •
- Protect KOVALTRY from extreme exposure to light and store the vial with the lyophilized powder in the carton prior to use.
Product After Reconstitution
- •
- Administer reconstituted KOVALTRY as soon as possible. If not, store at room temperature for no longer than 3 hours.
- •
- Do not use KOVALTRY if the reconstituted solution is cloudy or has particulate matter.
- •
- Use the administration set provided.
-
17 PATIENT COUNSELING INFORMATION
- •
- Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Hypersensitivity reactions are possible with KOVALTRY [see Warnings and Precautions (5.1)]. Warn patients of the early signs of hypersensitivity reactions (including tightness of the chest or throat, dizziness, mild hypotension and nausea during infusion) which can progress to anaphylaxis. Advise patients to discontinue use of the product if these symptoms occur and seek immediate emergency treatment with resuscitative measures such as the administration of epinephrine and oxygen.
- •
- Inhibitor formation may occur at any time in the treatment of a patient with hemophilia A [see Warnings and Precautions (5.2)]. Advise patients to contact their physician or treatment center for further treatment and/or assessment, if they experience a lack of clinical response to Factor VIII replacement therapy, as this may be a manifestation of an inhibitor.
-
PATIENT PACKAGE INSERT
FDA-Approved Patient Labeling
Patient Information
KOVALTRY (KOH-vahl-tree)
Antihemophilic Factor (Recombinant)
This leaflet summarizes important information about KOVALTRY with vial adapter. Please read it carefully before using this medicine. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about KOVALTRY. If you have any questions after reading this, ask your healthcare provider.
Do not attempt to self-infuse unless you have been taught how by your healthcare provider or hemophilia center.
What is KOVALTRY?
KOVALTRY is a medicine used to replace clotting factor (Factor VIII or antihemophilic factor) that is missing in people with hemophilia A (also called “classic” hemophilia). Hemophilia A is an inherited bleeding disorder that prevents blood from clotting normally.
KOVALTRY is used to treat and control bleeding in adults and children with hemophilia A. Your healthcare provider may give you KOVALTRY when you have surgery. KOVALTRY can reduce the number of bleeding episodes in adults and children with hemophilia A when used regularly (prophylaxis).
KOVALTRY is not used to treat von Willebrand Disease.
Who should not use KOVALTRY?
You should not use KOVALTRY if you
- •
- are allergic to rodents (like mice and hamsters).
- •
- are allergic to any ingredients in KOVALTRY.
What should I tell my healthcare provider before I use KOVALTRY?
- •
- Tell your healthcare provider about all of your medical conditions.
- •
- Tell your healthcare provider and pharmacist about all of the medicines you take, including all prescription and non-prescription medicines, such as over-the-counter medicines, supplements, or herbal remedies.
- •
- Tell your healthcare provider if you have been told you have heart disease or are at risk for heart disease.
- •
- Tell your healthcare provider if you have been told that you have inhibitors to Factor VIII (because KOVALTRY may not work for you).
What are the possible side effects of KOVALTRY?
The common side effects of KOVALTRY are fever, headache, and rash, in addition to inhibitors in patients who were not previously treated or minimally treated with Factor VIII products.
Your body may make antibodies, called “inhibitors” against KOVALTRY, which may stop KOVALTRY from working properly. If your bleeding is not adequately controlled, it could be due to the development of Factor VIII inhibitors. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for the development of inhibitors to Factor VIII.
Allergic reactions may occur with KOVALTRY. Call your healthcare provider right away and stop treatment if you get tightness of the chest or throat, dizziness, decrease in blood pressure, and nausea.
These are not all the possible side effects with KOVALTRY. You can ask your healthcare provider for information that is written for healthcare professionals.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
What are the KOVALTRY dosage strengths?
KOVALTRY with 2.5 mL or 5 mL Sterile Water for Injection (SWFI) comes in five different dosage strengths labeled as International Units (IU): 250 IU, 500 IU, 1000 IU, 2000 IU, and 3000 IU. The five different strengths are color-coded as follows:
Blue
250 IU with 2.5 mL SWFI
Green
500 IU with 2.5 mL SWFI
Red
1000 IU with 2.5 mL SWFI
Yellow
2000 IU with 5 mL SWFI
Gray
3000 IU with 5 mL SWFI
How do I store KOVALTRY?
Do not freeze KOVALTRY.
Store KOVALTRY at +2°C to +8°C (36°F to 46°F) for up to 30 months from the date of manufacture. Within this period, KOVALTRY may be stored for a period of up to 12 months at temperatures up to +25°C or 77°F.
Record the starting date of room temperature storage clearly on the unopened product carton. Once stored at room temperature, do not return the product to the refrigerator. The product then expires after storage at room temperature for 12 months, or after the expiration date on the product vial, whichever is earlier. Store vials in their original carton and protect them from extreme exposure to light.
Administer reconstituted KOVALTRY as soon as possible. If not, store at room temperature for no longer than 3 hours.
Throw away any unused KOVALTRY after the expiration date.
Do not use reconstituted KOVALTRY if it is not clear.
What else should I know about KOVALTRY and hemophilia A?
Finding veins for injections may be difficult in young children. When frequent injections are required, your healthcare provider may propose to have a device surgically placed under the skin to facilitate access to the bloodstream. These devices may result in infections.
Medicines are sometimes prescribed for purposes other than those listed here. Do not use KOVALTRY for a condition for which it is not prescribed. Do not share KOVALTRY with other people, even if they have the same symptoms that you have.
This leaflet summarizes the most important information about KOVALTRY. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider or pharmacist for information about KOVALTRY that was written for healthcare professionals.
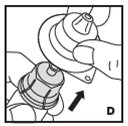
Instructions for Use
KOVALTRY (KOH-vahl-tree)
Antihemophilic Factor (Recombinant)
Do not attempt to self-infuse unless you have been taught how by your healthcare provider or hemophilia center.
You should always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using KOVALTRY. If you are unsure of the procedures, please call your healthcare provider before using.
Call your healthcare provider right away if bleeding is not controlled after using KOVALTRY.
Your healthcare provider will prescribe the dose that you should take.
Your healthcare provider may need to take blood tests from time to time.
Talk to your healthcare provider before traveling. You should plan to bring enough KOVALTRY for your treatment during this time.
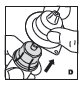
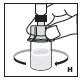
See the step-by-step instructions below for reconstituting KOVALTRY with vial adapter. Follow the specific infusion instruction leaflet included with the infusion set provided.
Carefully handle KOVALTRY. Dispose of all materials, including any leftover reconstituted KOVALTRY product, in an appropriate container.
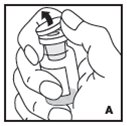
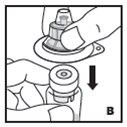
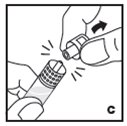
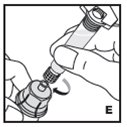
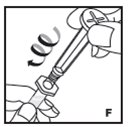
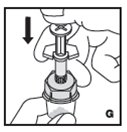
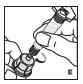
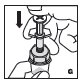
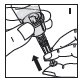
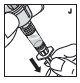
Reconstitution
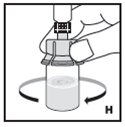
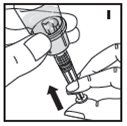
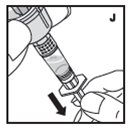
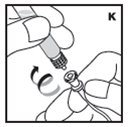
Always work on a clean surface and wash your hands before performing the following procedure. Use only the components for reconstitution and administration that are provided with each package of KOVALTRY. If a package is opened or damaged, do not use this component. If these components cannot be used, please contact your healthcare provider.
Prepare a clean flat surface and gather all the materials needed for the infusion.
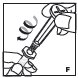
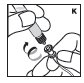
Pooling
If the dose requires more than one vial, reconstitute each vial as described above with the diluent syringe provided. To combine the content of the vials, use a larger plastic syringe (not provided) to pool the solution into the syringe and administer as usual.
Rate of Administration
The entire dose of KOVALTRY can usually be infused within 1 to 15 minutes. Your healthcare provider will determine the rate of administration that is best for you.
Resources at Bayer available to the patient:
For Adverse Reaction Reporting, contact Bayer Medical Communications 1-888-84-BAYER (1-888-842-2937)
To receive more product information, contact KOVALTRY Customer Service 1-888-606-3780
Bayer Reimbursement HELPline 1-800-288-8374
For more information, visit www.KOVALTRY-us.com
Bayer HealthCare LLC
Whippany, NJ 07981 USAU.S. License No. 8
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration. Revised: 10/2021
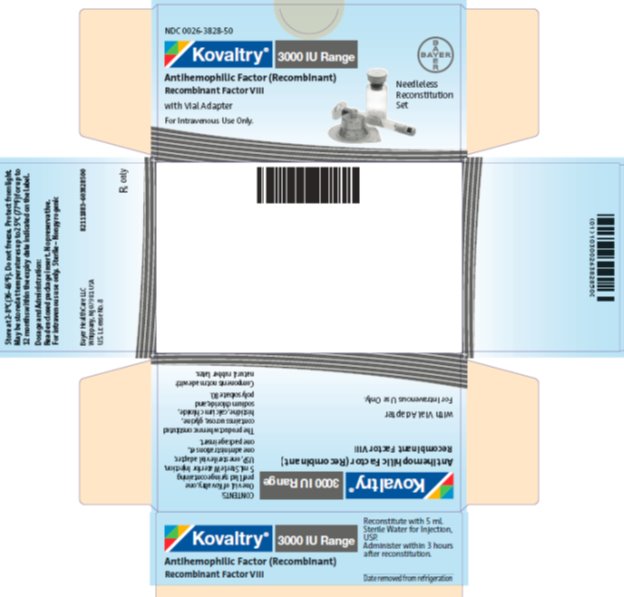
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KOVALTRY
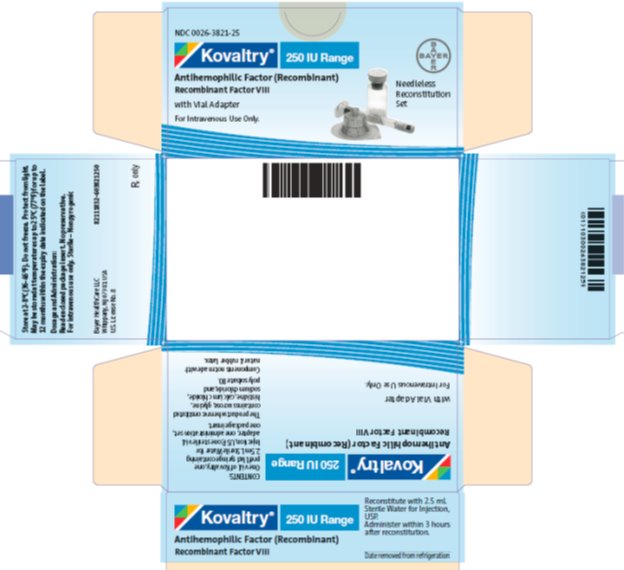
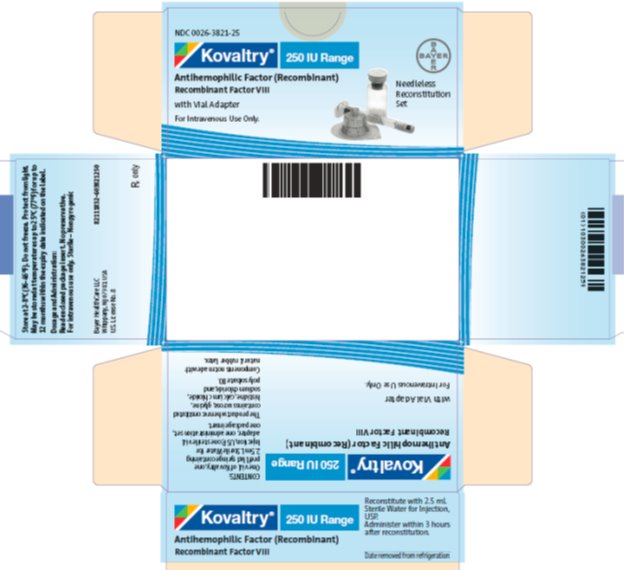
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0026-3821 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-3821-25 1 in 1 BOX; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0026-3821-99 1 in 1 BOX; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 2.5 mL Part 2 1 SYRINGE 2.5 mL Part 1 of 2 KOVALTRY
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0026-4821 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 250 [iU] in 2.5 mL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) SODIUM CHLORIDE (UNII: 451W47IQ8X) GLYCINE (UNII: TE7660XO1C) HISTIDINE (UNII: 4QD397987E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-4821-01 2.5 mL in 1 VIAL, SINGLE-USE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:0026-4821-99 2.5 mL in 1 VIAL, SINGLE-USE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Part 2 of 2 DILUENT
water solutionProduct Information Item Code (Source) NDC:0026-0426 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-0426-02 2.5 mL in 1 SYRINGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 KOVALTRY
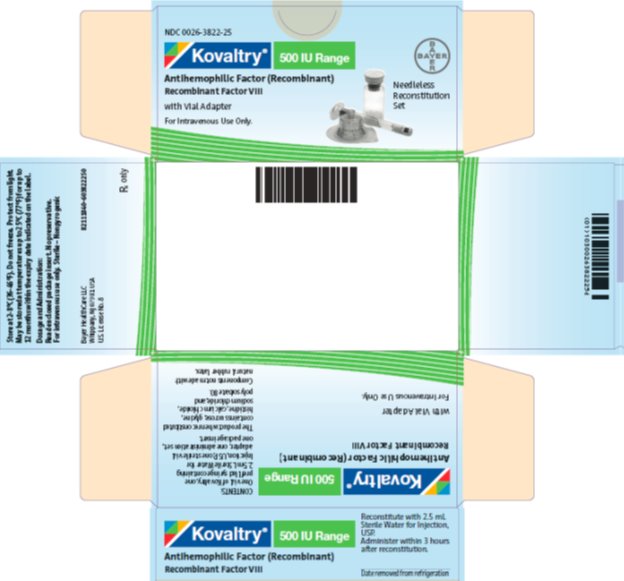
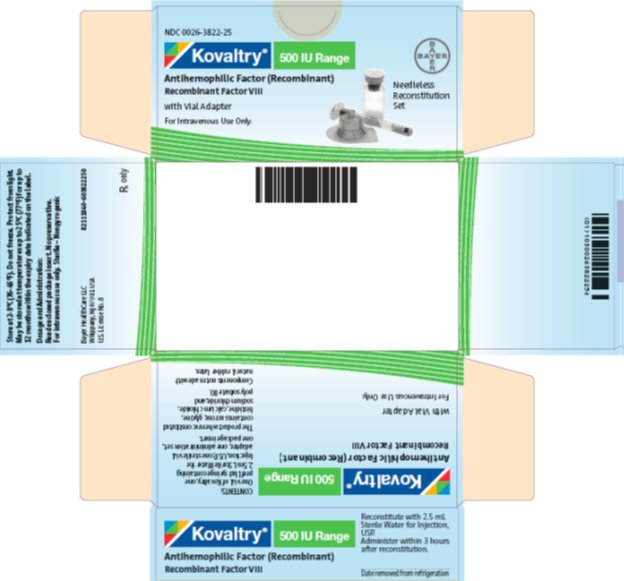
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0026-3822 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-3822-25 1 in 1 BOX; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0026-3822-99 1 in 1 BOX; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 2.5 mL Part 2 1 SYRINGE 2.5 mL Part 1 of 2 KOVALTRY
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0026-4822 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 500 [iU] in 2.5 mL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) GLYCINE (UNII: TE7660XO1C) HISTIDINE (UNII: 4QD397987E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SUCROSE (UNII: C151H8M554) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-4822-01 2.5 mL in 1 VIAL, SINGLE-USE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0026-4822-99 2.5 mL in 1 VIAL, SINGLE-USE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Part 2 of 2 DILUENT
water solutionProduct Information Item Code (Source) NDC:0026-0426 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-0426-02 2.5 mL in 1 SYRINGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 KOVALTRY
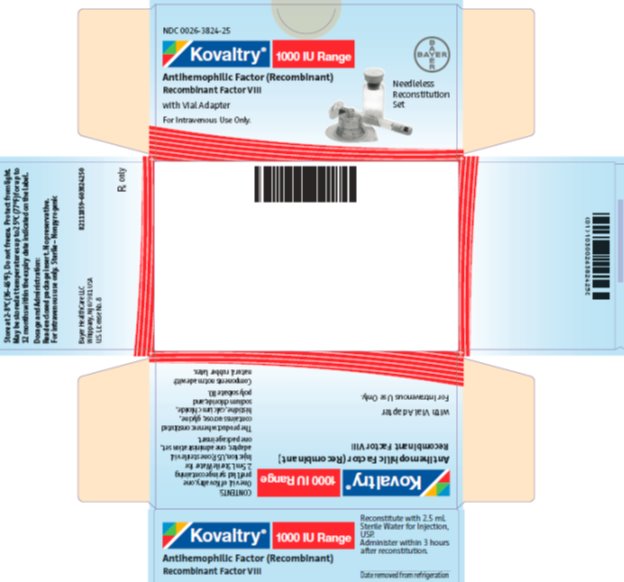
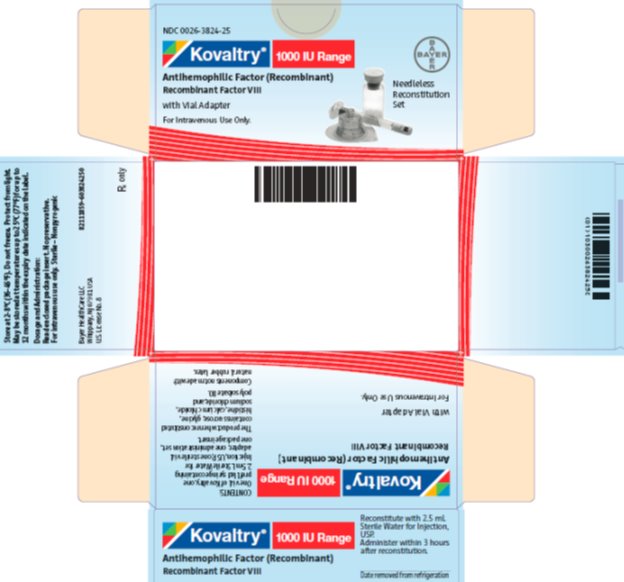
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0026-3824 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-3824-25 1 in 1 BOX; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0026-3824-99 1 in 1 BOX; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 2.5 mL Part 2 1 SYRINGE 2.5 mL Part 1 of 2 KOVALTRY
antihemphilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0026-4824 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 1000 [iU] in 2.5 mL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) GLYCINE (UNII: TE7660XO1C) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SUCROSE (UNII: C151H8M554) HISTIDINE (UNII: 4QD397987E) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-4824-01 2.5 mL in 1 VIAL, SINGLE-USE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0026-4824-99 2.5 mL in 1 VIAL, SINGLE-USE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Part 2 of 2 DILUENT
water solutionProduct Information Item Code (Source) NDC:0026-0426 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-0426-02 2.5 mL in 1 SYRINGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 KOVALTRY
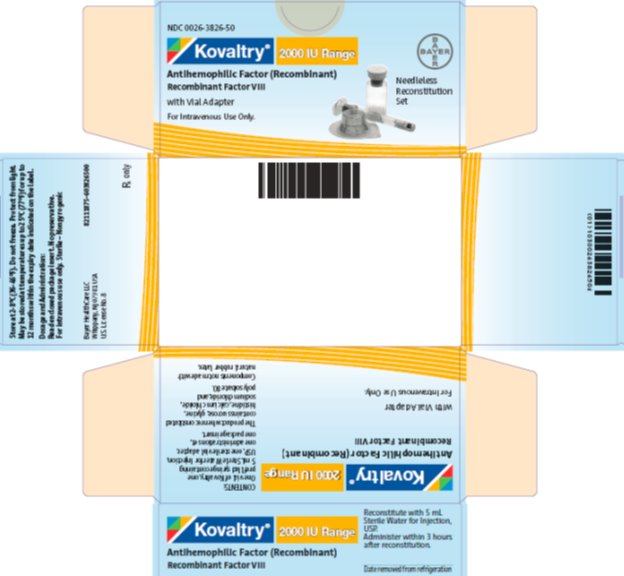
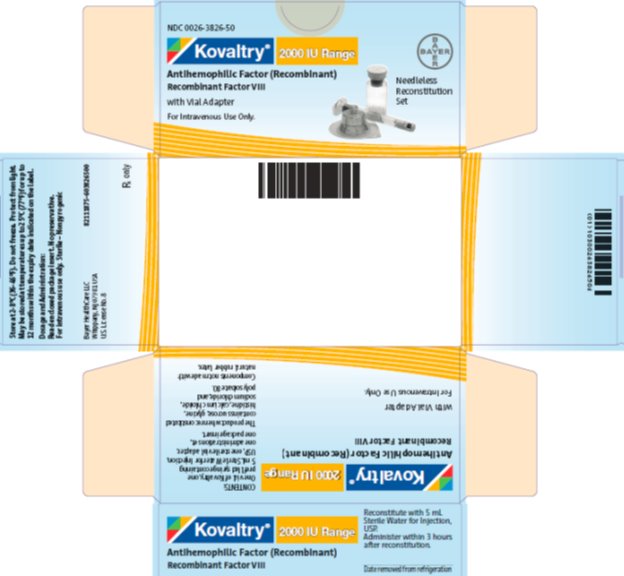
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0026-3826 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-3826-50 1 in 1 BOX; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0026-3826-99 1 in 1 BOX; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 5 mL Part 2 1 SYRINGE 5 mL Part 1 of 2 KOVALTRY
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0026-4826 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 2000 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) GLYCINE (UNII: TE7660XO1C) HISTIDINE (UNII: 4QD397987E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SUCROSE (UNII: C151H8M554) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-4826-01 5 mL in 1 VIAL, SINGLE-USE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC:0026-4826-99 5 mL in 1 VIAL, SINGLE-USE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Part 2 of 2 DILUENT
water solutionProduct Information Item Code (Source) NDC:0026-0426 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-0426-05 5 mL in 1 SYRINGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0026-0426-15 5 mL in 1 SYRINGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 KOVALTRY
antihemophilic factor (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0026-3828 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-3828-50 1 in 1 BOX; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0026-3828-99 1 in 1 BOX; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 5 mL Part 2 1 SYRINGE 5 mL Part 1 of 2 KOVALTRY
antihemophilic factor (recombinant) injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0026-4828 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT (UNII: P89DR4NY54) (ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT - UNII:P89DR4NY54) ANTIHEMOPHILIC FACTOR, HUMAN RECOMBINANT 3000 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) GLYCINE (UNII: TE7660XO1C) HISTIDINE (UNII: 4QD397987E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-4828-01 5 mL in 1 VIAL, SINGLE-USE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0026-4828-99 5 mL in 1 VIAL, SINGLE-USE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Part 2 of 2 DILUENT
water solutionProduct Information Item Code (Source) NDC:0026-0426 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0026-0426-05 5 mL in 1 SYRINGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:0026-0426-15 5 mL in 1 SYRINGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125574 03/17/2016 Labeler - Bayer HealthCare LLC (127769128) Establishment Name Address ID/FEI Business Operations Bayer HealthCare LLC 127769128 MANUFACTURE(0026-3821, 0026-4821, 0026-0426, 0026-3822, 0026-4822, 0026-0426, 0026-3824, 0026-4824, 0026-0426, 0026-3826, 0026-4826, 0026-0426, 0026-3828, 0026-4828, 0026-0426) , API MANUFACTURE(0026-3821, 0026-4821, 0026-0426, 0026-3822, 0026-4822, 0026-0426, 0026-3824, 0026-4824, 0026-0426, 0026-3826, 0026-4826, 0026-0426, 0026-3828, 0026-4828, 0026-0426)