Label: QALSODY- tofersen injection
- NDC Code(s): 64406-109-01
- Packager: Biogen Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use QALSODY ™ safely and effectively. See full prescribing information for QALSODY. QALSODY (tofersen) injection, for intrathecal use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
QALSODY is indicated for the treatment of amyotrophic lateral sclerosis (ALS) in adults who have a mutation in the superoxide dismutase 1 (SOD1) gene. This indication is approved under accelerated ...
-
2 DOSAGE AND ADMINISTRATION
2.1. Dosing Information - Recommended Dosage - Administer QALSODY intrathecally using a lumbar puncture by, or under the direction of, healthcare professionals experienced in performing ...
-
3 DOSAGE FORMS AND STRENGTHS
Injection: 100 mg/15 mL (6.7 mg/mL) as a clear and colorless to slightly yellow solution in a single-dose vial.
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
5.1. Myelitis and/or Radiculitis - Serious adverse reactions of myelitis and radiculitis have been reported in patients treated with QALSODY. Six patients treated with QALSODY experienced ...
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed elsewhere in the labeling: Myelitis and/or Radiculitis [see Warnings and Precautions (5.1)] Papilledema and Elevated ...
-
8 USE IN SPECIFIC POPULATIONS
8.1. Pregnancy - Risk Summary - There are no adequate data on developmental risks associated with the use of QALSODY in pregnant women to evaluate for a drug-associated risk of major birth ...
-
11 DESCRIPTION
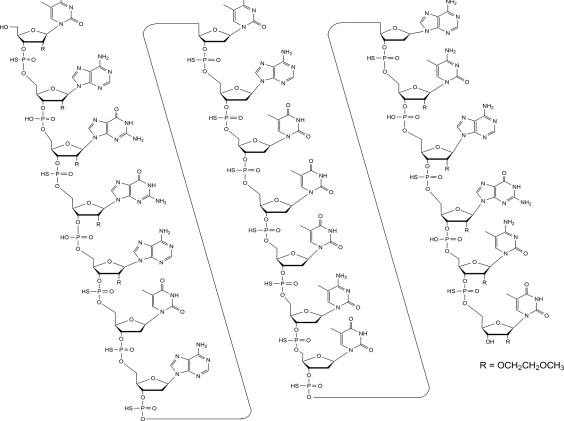
Tofersen, an antisense oligonucleotide, is a 20-base residue (20-mer) 5-10-5 MOE gapmer mixed backbone oligonucleotide. Of the nineteen internucleotide linkages, fifteen are 3′-O to 5′-O ...
-
12 CLINICAL PHARMACOLOGY
12.1. Mechanism of Action - Tofersen is an antisense oligonucleotide that causes degradation of SOD1 mRNA through binding to SOD1 mRNA, which results in a reduction of SOD1 protein ...
-
13 NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Studies to assess the carcinogenic potential of tofersen have not been conducted. Mutagenesis - Tofersen was ...
-
14 CLINICAL STUDIES
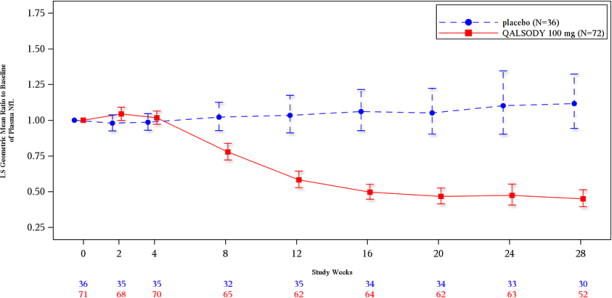
The efficacy of QALSODY was assessed in a 28-week randomized, double-blind, placebocontrolled clinical study in patients 23 to 78 years of age with weakness attributable to ALS and a SOD1 mutation ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1. How Supplied - QALSODY injection is a sterile, clear and colorless to slightly yellow solution supplied as 100 mg/15 mL (6.7 mg/mL) solution in a single-dose glass vial free of ...
-
17 PATIENT COUNSELING INFORMATION
Myelitis and/or Radiculitis - Inform patients and caregivers that QALSODY could cause myelitis and radiculitis. Instruct patients and caregivers to contact their healthcare provider if symptoms ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Carton Label - 1 vial - NDC: 64406-109-01 - QALSODY™ (tofersen) Injection - 100 mg/15 mL - (6.7 mg/mL) Sterile solution for - Intrathecal Injection Only - Rx ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Vial Label - QALSODY™ (tofersen) Injection - 100 mg/15 mL - (6.7 mg/mL) Sterile solution for Intrathecal Injection Only - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information