Buprenorphine Sublingual Tablets CIII
(BUE-pre-NOR-feen)
|
IMPORTANT:
Keep buprenorphine sublingual tablets in a secure place away from children. Accidental use by a child is a medical emergency and can result in death. If a child accidentally uses buprenorphine sublingual tablets, get emergency help right away.
|
Read this Medication Guide that comes with buprenorphine sublingual tablets before you start taking them and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor. Talk to your doctor or pharmacist if you have questions about buprenorphine sublingual tablets.
Share the important information in this Medication Guide with members of your household.
|
What is the most important information I should know about buprenorphine sublingual tablets?
- Buprenorphine is a medicine in buprenorphine sublingual tablets that can cause serious and life-threatening problems, especially if you take or use certain other medicines or drugs. Call your healthcare provider right away or get emergency help if you: feel faint or dizzy
- cannot think well or clearly
- have mental changes such as confusion
- have slowed reflexes
- have slower breathing than you normally have
- have a high body temperature
- have severe sleepiness
- feel agitated o have blurred vision
- have stiff muscles
- have problems with coordination
- have trouble walking o have slurred speech
These can be signs of an overdose or other serious problems.
- Do not switch from buprenorphine sublingual tablets to other medicines that contain buprenorphine without talking with your doctor. The amount of buprenorphine in a dose of buprenorphine sublingual tablets is not the same as the amount of buprenorphine in other medicines that contain buprenorphine. Your doctor will prescribe a starting dose of buprenorphine sublingual tablets that may be different than other buprenorphine containing medicines you may have been taking.
- Buprenorphine sublingual tablets contain an opioid that can cause physical dependence.
- Do not stop taking buprenorphine sublingual tablets without talking to your doctor. You could become sick with uncomfortable withdrawal signs and symptoms because your body has become used to this medicine.
- Physical dependence is not the same as drug addiction.
- Buprenorphine sublingual tablets are not for occasional or “as needed” use.
- An overdose and even death can happen if you take benzodiazepines, sedatives, tranquilizers, antidepressants, or alcohol while using buprenorphine sublingual tablets. Ask your doctor what you should do if you are taking one of these.
- Call a doctor or get emergency help right away if you:
- Feel sleepy and uncoordinated
- Have blurred vision
- Have slurred speech
- Cannot think well or clearly
- Have slowed reflexes and breathing
- Do not inject (“shoot-up”) or snort buprenorphine sublingual tablets
- Injecting buprenorphine sublingual tablets may cause life-threatening infections and other serious health problems.
- Crushing and/or dissolving buprenorphine sublingual tablets and then injecting it (“shooting up”) could cause serious withdrawal symptoms such as pain, cramps, vomiting, diarrhea, anxiety, sleep problems, and cravings.
- Snorting buprenorphine sublingual tablets could cause severe withdrawal symptoms such as pain, cramps, and vomiting.
- In an emergency, have family members tell emergency department staff that you are physically dependent on an opioid and are being treated with buprenorphine sublingual tablets.
- Never give anyone else your buprenorphine sublingual tablets. They could die from taking it. Selling or giving away buprenorphine sublingual tablets is against the law.
- Store buprenorphine sublingual tablets securely, out of sight and reach of children, and in a location not accessible by others, including visitors to the home.
|
What are buprenorphine sublingual tablets?
- Buprenorphine sublingual tablets are a prescription medicine used to treat adults who are addicted to (dependent on) opioid drugs (either prescription or illegal), as part of a complete treatment program that also includes counseling and behavioral therapy.
- Buprenorphine sublingual tablets are most often used for the first 1 or 2 days to help you start with treatment.
|
Buprenorphine sublingual tablets are a controlled substance (CIII) because they contain buprenorphine, which can be a target for people who abuse prescription medicines or street drugs. Keep your buprenorphine sublingual tablets in a safe place to protect it from theft. Never give your buprenorphine sublingual tablets to anyone else; it can cause death or harm them. Selling or giving away this medicine is against the law.
|
- It is not known if buprenorphine sublingual tablets are safe or effective in children.
|
Who should not take buprenorphine sublingual tablets?
Do not take buprenorphine sublingual tablets if you are allergic to buprenorphine.
|
What should I tell my doctor before taking buprenorphine sublingual tablets?
Buprenorphine sublingual tablets may not be right for you. Before taking buprenorphine sublingual tablets, tell your doctor if you:
- Have liver or kidney problems
- Have trouble breathing or lung problems
- Have an enlarged prostate gland (men)
- Have a head injury or brain problem
- Have problems urinating
- Have a curve in your spine that affects your breathing
- Have gallbladder problems
- Have adrenal gland problems
- Have Addison’s disease
- Have low thyroid (hypothyroidism)
- Have a history of alcoholism
- Have mental problems such as hallucinations (seeing or hearing things that are not there)
- Have any other medical condition
- Are pregnant or plan to become pregnant. If you take buprenorphine sublingual tablets while pregnant, your baby may have signs of opioid withdrawal at birth. Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy. Talk to your doctor if you are pregnant or plan to become pregnant.
- Are breastfeeding or plan to breastfeed. Buprenorphine can pass into your milk and may harm your baby. Talk to your doctor about the best way to feed your baby if you take buprenorphine sublingual tablets. Monitor your baby for increased sleepiness and breathing problems.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Buprenorphine sublingual tablets may affect the way other medicines work, and other medicines may affect how buprenorphine sublingual tablets work. Some medicines may cause serious or life-threatening medical problems when taken with buprenorphine sublingual tablets.
Sometimes the doses of certain medicines and buprenorphine sublingual tablets may need to be changed if used together. Do not take any medicine while using buprenorphine sublingual tablets until you have talked with your doctor. Your doctor will tell you if it is safe to take other medicines while you are taking buprenorphine sublingual tablets.
Be especially careful about taking other medicines that may make you sleepy, such as muscle relaxants, pain medicines, tranquilizers, antidepressant medicines, sleeping pills, anxiety medicines or antihistamines.
Know the medicines you take. Keep a list of them to show your doctor or pharmacist each time you get a new medicine.
|
How should I take buprenorphine sublingual tablets?
- Always take buprenorphine sublingual tablets exactly as your doctor tells you
. Your doctor may change your dose after seeing how it affects you. Do not change your dose unless your doctor tells you to change it.
- Do not take buprenorphine sublingual tablets more often than prescribed by your doctor.
- If you are prescribed a dose of 2 or more buprenorphine sublingual tablets at the same time:
- Ask your doctor for instructions on the right way to take buprenorphine sublingual tablets
- Follow the same instructions every time you take a dose of buprenorphine sublingual tablets
- Put the tablets under your tongue. Let them dissolve completely.
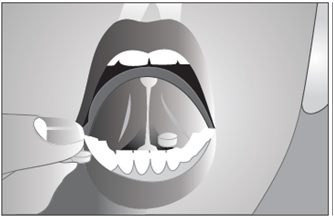
- While buprenorphine sublingual tablet is dissolving, do not chew or swallow the tablet because the medicine will not work as well.
- Talking while the tablet is dissolving can affect how well the medicine in buprenorphine sublingual tablet is absorbed.
- If you miss a dose of buprenorphine sublingual tablets, take your medicine when you remember. If it is almost time for your next dose, skip the missed dose and take the next dose at your regular time. Do not take 2 doses at the same time unless your doctor tells you to. If you are not sure about your dosing, call your doctor.
- Do not stop taking buprenorphine sublingual tablets suddenly. You could become sick and have withdrawal symptoms because your body has become used to the medicine. Physical dependence is not the same as drug addiction. Your doctor can tell you more about the differences between physical dependence and drug addiction. To have fewer withdrawal symptoms, ask your doctor how to stop using buprenorphine sublingual tablets the right way.
If you take too many buprenorphine sublingual tablets or overdose, call Poison Control or get emergency medical help right away.
|
What should I avoid while taking buprenorphine sublingual tablets?
-
Do not drive, operate heavy machinery, or perform any other dangerous activities until you know how this medication affects you. Buprenorphine can cause drowsiness and slow reaction times. This may happen more often in the first few weeks of treatment when your dose is being changed, but can also happen if you drink alcohol or take other sedative drugs when you take buprenorphine sublingual tablets.
-
You should not drink alcohol while using buprenorphine sublingual tablets, as this can lead to loss of consciousness or even death.
|
What are the possible side effects of buprenorphine sublingual tablets?
Buprenorphine sublingual tablets can cause serious side effects, including:
-
See “What is the most important information I should know about buprenorphine sublingual tablets?”
-
Respiratory problems. You have a higher risk of death and coma if you take buprenorphine sublingual tablets with other medicines, such as benzodiazepines.
-
Sleepiness, dizziness, and problems with coordination
-
Dependency or abuse
-
Liver problems. Call your doctor right away if you notice any of these signs of liver problems: Your skin or the white part of your eyes turning yellow (jaundice), urine turning dark, stools turning light in color, you have less of an appetite, or you have stomach (abdominal) pain or nausea. Your doctor should do tests before you start taking and while you take buprenorphine sublingual tablets.
-
Allergic reaction. You may have a rash, hives, swelling of the face, wheezing, or a loss of blood pressure and consciousness. Call a doctor or get emergency help right away.
-
Opioid withdrawal. This can include: shaking, sweating more than normal, feeling hot or cold more than normal, runny nose, watery eyes, goose bumps, diarrhea, vomiting, and muscle aches. Tell your doctor if you develop any of these symptoms.
-
Decrease in blood pressure. You may feel dizzy if you get up too fast from sitting or lying down.
Common side effects of buprenorphine sublingual tablets include:
- Nausea
- Vomiting
- Drug withdrawal syndrome
- Headache
- Sweating
- Numb mouth
- Constipation
- Swollen and/or painful tongue
- The inside of your mouth is more red than normal
- Intoxication (feeling lightheaded or drunk)
- Disturbance in attention
- Irregular heart beat (palpitations)
- Decrease in sleep (insomnia)
- Blurred vision
- Back pain
- Fainting
- Dizziness
- Sleepiness
Tell your doctor about any side effect that bothers you or that does not go away.
These are not all the possible side effects of buprenorphine sublingual tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
|
How should I store buprenorphine sublingual tablets?
- Store buprenorphine sublingual tablets at room temperature between 20° to 25°C (68° to 77°F). Protect from light.
-
Keep buprenorphine sublingual tablets in a safe place, out of the sight and reach of children.
- Retain blister card in carton until time of use.
|
How should I dispose of unused buprenorphine sublingual tablets?
- Dispose of unused buprenorphine sublingual tablets as soon as you no longer need them.
- Dispose of expired, unwanted or unused buprenorphine sublingual tablets by promptly flushing down the toilet, if a drug take-back option is not readily available. Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
|
General information about the safe and effective use of buprenorphine sublingual tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take buprenorphine sublingual tablets for a condition for which it was not prescribed. Do not give buprenorphine sublingual tablets to other people, even if they have the same symptoms you have. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about buprenorphine sublingual tablets. If you would like more information, talk to your doctor or pharmacist. You can ask your doctor or pharmacist for information that is written for health professionals.
For more information call 1-800-818-4555.
|
What are the ingredients in buprenorphine sublingual tablets?
Active Ingredient: buprenorphine hydrochloride
Inactive Ingredients: lactose monohydrate, mannitol, povidone, anhydrous citric acid, sodium citrate, butylated hydroxyanisole, corn starch, pregelatinized starch, and magnesium stearate
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
Manufactured by:
Sun Pharmaceutical Industries Limited.
Survey No. 259/15,
Dadra-396 191, (U.T. of D & NH), India.
PGPI0257E
|
