EMBEDA™ (im-bed-a)
(morphine sulfate and
naltrexone hydrochloride)
Extended Release Capsules CII
IMPORTANT: Keep EMBEDA in a safe place away from children. Accidental use by a child is a medical emergency and can result in death. If a child accidentally takes EMBEDA, get emergency help right away.
Read the Medication Guide that comes with EMBEDA before you start taking it and each time you get a new prescription. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. Share this important information with members of your household.
What Is the Most Important Information I Should Know About EMBEDA?
-
Do not crush, dissolve, or chew EMBEDA capsules or the
capsule contents before swallowing. If EMBEDA is taken in this way, both the
morphine and naltrexone in EMBEDA will be released too fast. This is dangerous. It may cause you to have trouble breathing, and
lead to death.
- If your body is not used to taking opioids and your body absorbs too much morphine, you could overdose and die.
- If you have been taking opioids (narcotics) for a period of time, and your body absorbs the naltrexone in EMBEDA, this could cause you to have uncomfortable withdrawal symptoms.
- Take EMBEDA exactly as prescribed by your healthcare provider.
- EMBEDA is not for use to treat pain that you only have once in a while (“as needed”).
- If you cannot swallow capsules, tell your healthcare provider. There may be another way to take EMBEDA that may be right for you. See “How should I take EMBEDA?”
- Do not take the highest dose of EMBEDA (morphine sulfate 100 mg and naltrexone hydrochloride 4 mg) unless you are “opioid tolerant.” Opioid tolerant means that you regularly use another opioid medicine for your constant (around the clock) pain and your body is used to it.
- Do not drink alcohol, or use prescription or non-prescription medicines that contain alcohol while you are being treated with EMBEDA. Alcohol can cause very high levels of morphine in your blood and you can die due to an overdose of morphine.
- Prevent theft, misuse or abuse. Keep EMBEDA in a safe place to protect it from being stolen. EMBEDA can be a target for people who misuse or abuse prescription medicines or street drugs.
- Never give EMBEDA to anyone else, even if they have the same symptoms you have. It may harm them or even cause death.
See the section “What are the possible side effects of EMBEDA?” for more information about side effects.
What is EMBEDA?
- EMBEDA is a prescription medicine that contains morphine sulfate, an opioid receptor agonist (narcotic pain medicine) and naltrexone hydrochloride, an opioid receptor antagonist. Naltrexone hydrochloride is in the middle of each pellet and has a special coating to protect it from being released. If you crush or chew EMBEDA, the naltrexone will be released all at one time. See “What is the most important information I should know about EMBEDA?
- EMBEDA is a federally controlled substance (CII) because it is a strong opioid pain medicine that can be abused by people who abuse prescription medicines or street drugs.
- EMBEDA is used to manage moderate to severe pain that continues around-the-clock and is expected to last for a long period of time.
- It is not known if EMBEDA is safe and works in children under the age of 18.
Who Should Not Take EMBEDA?
Do not take EMBEDA if you:
- are having an asthma attack or have severe asthma, trouble breathing, or lung problems.
- have a bowel blockage called paralytic ileus.
- are allergic to morphine, morphine salts, naltrexone, or any of the ingredients in EMBEDA. See the end of this Medication Guide for a complete list of ingredients in EMBEDA.
What Should I Tell My Healthcare Provider Before Starting EMBEDA?
-
EMBEDA may not be right for you. Tell your healthcare
provider about all of your medical conditions, especially if you:
- have trouble breathing or lung problems
- have a head injury or brain problem
- have liver or kidney problems
- have adrenal gland problems, such as Addison’s disease
- have convulsions or seizures
- have thyroid problems
- have problems urinating or prostate problems
- have constipation or other bowel problems
- have problems with your pancreas or gallbladder
- have severe scoliosis
- have a drinking problem or alcoholism
- have severe mental problems or hallucinations (see or hear things that are not really there)
- have or have had drug abuse or drug addiction problems
- are planning to have surgery (cordotomy) or another procedure that will interrupt the pain signals to your body.
- are pregnant or plan to become pregnant. EMBEDA may harm your unborn baby.
- are breastfeeding. EMBEDA may pass through your milk and may harm your baby. You should not breastfeed while taking EMBEDA.
- Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may cause serious problems when taken with EMBEDA. Sometimes, the doses of certain medicines and EMBEDA may need to be changed if used together.
- Be especially careful about taking other medicines that make you sleepy such
as:
- other pain medicines
- anti-depressant medicines
- sleeping pills
- anti-anxiety medicines
- muscle relaxants
- antihistamines
- anti-nausea medicines
- tranquilizers
Also tell your healthcare provider if you take:
- cimetidine (Tagamet)
- a water pill (diuretic)
- an anticholinergic medicine
- Do not take EMBEDA if you already take a monoamine oxidase inhibitor medicine (MAOI) or within 14 days after you stop taking an MAOI medicine.
- Do not take any new medicine while using EMBEDA until you have talked to your healthcare provider or pharmacist. They will tell you if it is safe to take other medicines with EMBEDA.
Ask your healthcare provider if you are not sure if your medicine is one listed above.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How Should I Take EMBEDA?
- Take EMBEDA exactly as prescribed by your healthcare provider. Do not change your dose unless your healthcare provider tells you to.
- You can take EMBEDA with or without food.
- Swallow EMBEDA capsule whole. Do not crush, dissolve, or chew EMBEDA or the pellets in the capsules before swallowing. See “What is the most important information I should know about EMBEDA?”
- If you cannot swallow capsules, tell your healthcare provider. There may be another way to take EMBEDA that may be right for you. If your doctor tells you that you can take EMBEDA using this other way, follow these steps:
EMBEDA can be opened and the pellets inside the capsule can be sprinkled over apple sauce, as follows:
- Open the EMBEDA capsule and sprinkle the pellets over approximately one
tablespoon of apple sauce (Figure 1).
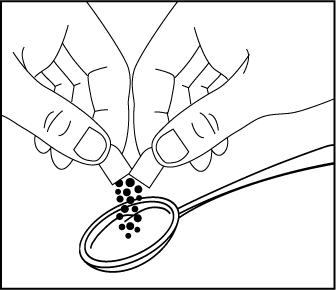 Figure 1 Swallow all of the apple sauce and pellets right away. Do not save any of
the apple sauce and pellets for another dose (Figure
2).
Figure 1 Swallow all of the apple sauce and pellets right away. Do not save any of
the apple sauce and pellets for another dose (Figure
2).
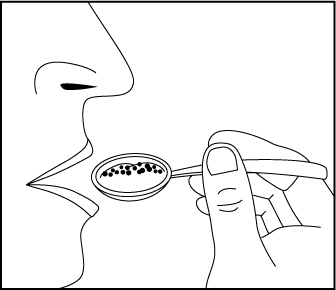 Figure 2 Rinse your mouth to make sure you have swallowed all of the pellets. Do not
chew the pellets (Figure 3).
Figure 2 Rinse your mouth to make sure you have swallowed all of the pellets. Do not
chew the pellets (Figure 3).
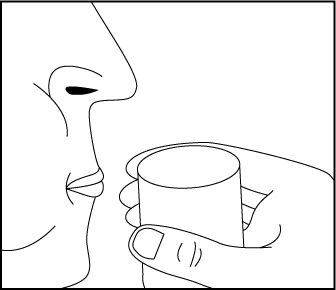 Figure 3 Flush the empty capsule down the toilet right away (Figure 4).
Figure 3 Flush the empty capsule down the toilet right away (Figure 4).
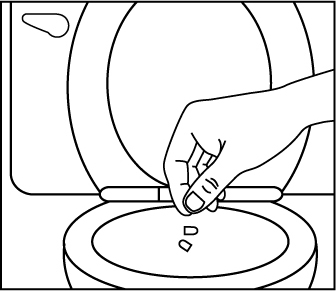 Figure 4 You should not receive EMBEDA through a nasogastric tube or gastric tube
(stomach tube).
Figure 4 You should not receive EMBEDA through a nasogastric tube or gastric tube
(stomach tube).
- If you miss a dose, take it as soon as possible. If it is almost time for your next dose, skip the missed dose. Just take the next dose at your regular time. Do not take 2 doses at the same time unless your healthcare provider tells you to. If you are not sure about your dosing, call your healthcare provider.
- If you take too much EMBEDA or overdose, call 911 or poison control center right away.
- Call your healthcare provider if the dose of EMBEDA that you are taking does not relieve your pain.
What Should I Avoid While Taking EMBEDA?
- Do not drive, operate heavy machinery, or do other dangerous activities, especially when you start taking EMBEDA and when your dose is changed, until you know how you react to this medicine. EMBEDA can make you sleepy, and also cause you to feel dizzy and lightheaded. Ask your healthcare provider to tell you when it is okay to do these activities.
What are the Possible Side Effects of EMBEDA?
EMBEDA can cause serious side effects, including:
- See “What is the most important information I should know about EMBEDA?”
-
EMBEDA can cause serious breathing problems that can become
life-threatening, especially if used the wrong way.
Call your healthcare provider or get medical help right away if:
- your breathing slows down
- you have shallow breathing (little chest movement with breathing)
- you feel faint, dizzy, confused, or
- have any other unusual symptoms
These can be symptoms that you have taken too much EMBEDA (overdose) or the dose is too high for you. These symptoms may lead to serious problems or death if not treated right away.
- EMBEDA can cause your blood pressure to drop. This can make you feel dizzy and faint if you get up too fast from sitting or lying down. Low blood pressure is also more likely to happen if you take other medicines that can also lower your blood pressure. Severe low blood pressure can happen if you lose blood or take certain other medicines.
- EMBEDA can cause physical dependence. Do not stop taking EMBEDA or any other opioid without talking to your healthcare provider. You could become sick with uncomfortable withdrawal symptoms because your body has become used to these medicines. Physical dependence is not the same as drug addiction.
- There is a chance of abuse or addiction with EMBEDA. The chance is higher if you are or have been addicted to or abused other medicines, street drugs, or alcohol, or if you have a history of mental problems.
-
Serious allergic reactions. Rarely, severe allergic
reactions happen in people who take a long-acting morphine medicine that is like
EMBEDA. Get medical help right away if you have any of these symptoms of a
severe allergic reaction:
- feel dizzy or faint
- trouble breathing
- pounding heart beat
- chest pain
- swelling of the face, throat, or tongue
- feeling of doom
The most common side effects of EMBEDA are
- constipation
- nausea
- sleepiness
- vomiting
- dizziness
- itching
- headache
These side effects may decrease with continued use. Talk to your healthcare provider if you continue to have these side effects. These are not all the possible side effects of EMBEDA. For a complete list, ask your healthcare provider or pharmacist.
Constipation (not often enough or hard bowel movements) is a common side effect of pain medicines (opioids) including EMBEDA and is unlikely to go away without treatment. Talk to your healthcare provider about dietary changes, and the use of laxatives (medicines to treat constipation) and stool softeners to prevent or treat constipation while taking EMBEDA.
Talk to your healthcare provider if you have any side effects that bother you or do not go away.
These are not all the possible side effects of EMBEDA. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
- Selling or giving away this medicine is against the law
How should I store EMBEDA?
- See “What is the most important information I should know about EMBEDA?"
- Keep EMBEDA out of the reach of children.
- Keep EMBEDA in the container it comes in.
- Keep EMBEDA at room temperature between 59° to 86°F (15° to 30°C).
- After you stop taking EMBEDA, flush the unused capsules down the toilet.
General Information about EMBEDA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use EMBEDA for conditions for which it was not prescribed. Do not give EMBEDA to other people, even if they have the same symptoms you have. It may harm them and even cause death. Sharing EMBEDA is against the law.
This medication guide summarizes the most important information about EMBEDA. If you would like more information, talk with your healthcare provider. Also, you can ask your pharmacist or healthcare provider for information about EMBEDA that is written for healthcare professionals. For more information call 1-800-776-3637 or go to www.kingpharm.com.
What are the ingredients in EMBEDA?
Active Ingredients: pellets of morphine sulfate and naltrexone hydrochloride
Inactive Ingredients common to all strengths: talc, ammonio methacrylate copolymer, sugar spheres, ethylcellulose, sodium chloride, polyethylene glycol, hydroxypropyl cellulose, dibutyl sebacate, methacrylic acid copolymer, diethyl phthalate, magnesium stearate, sodium lauryl sulfate, and ascorbic acid. The capsule shells contain gelatin, titanium dioxide, and grey ink, D and C yellow #10 (EMBEDA 20 mg/0.8 mg), FD and C red #3, FD and C blue #1 (EMBEDA 30 mg/1.2 mg), D and C red #28, FD and C red #40, FD and C blue #1 (EMBEDA 50 mg/2 mg), D and C red #28, FD and C red #40, FD and C blue #1 (EMBEDA 60 mg/2.4 mg), FD and C blue #1, FD and C red #40, FD and C yellow #6 (EMBEDA 80 mg/3.2 mg), D and C yellow #10, FD and C blue #1 (EMBEDA 100 mg/4 mg).
Manufactured for: King Pharmaceuticals, Inc., 501 Fifth Street, Bristol, TN 37620
(Telephone: 1-800-776-3637)
by: Actavis Elizabeth LLC, 200 Elmora Avenue, Elizabeth, NJ 07207 USA
EMBEDA is a trademark of Alpharma Pharmaceuticals LLC, a wholly owned subsidiary of King Pharmaceuticals, Inc.
41-1126
Revision: June 2009
This Medication Guide has been approved by the U.S. Food and Drug Administration
v.10