MEDICATION GUIDE
ADVAIR [ad′ vair] DISKUS® 100/50
(fluticasone propionate 100 mcg and salmeterol 50 mcg inhalation powder)
ADVAIR DISKUS® 250/50
(fluticasone propionate 250 mcg and salmeterol 50 mcg inhalation powder)
ADVAIR DISKUS® 500/50
(fluticasone propionate 500 mcg and salmeterol 50 mcg inhalation powder)
Read the Medication Guide that comes with ADVAIR DISKUS before you start using it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about ADVAIR DISKUS?
-
ADVAIR DISKUS contains 2 medicines:
- fluticasone propionate (the same medicine found in FLOVENT®), an inhaled corticosteroid medicine. Inhaled corticosteroids help to decrease inflammation in the lungs. Inflammation in the lungs can lead to asthma symptoms.
- salmeterol (the same medicine found in SEREVENT®), a long-acting beta2-agonist medicine or LABA. LABA medicines are used in patients with asthma and chronic obstructive pulmonary disease (COPD). LABA medicines help the muscles around the airways in your lungs stay relaxed to prevent symptoms, such as wheezing and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe. In severe cases, wheezing can stop your breathing and cause death if not treated right away.
- In patients with asthma, LABA medicines, such as salmeterol (one of the medicines in ADVAIR DISKUS), may increase the chance of death from asthma problems. In a large asthma study, more patients who used salmeterol died from asthma problems compared with patients who did not use salmeterol. It is not known whether fluticasone propionate, the other medicine in ADVAIR DISKUS, changes your chance of death from asthma problems seen with salmeterol. Talk with your healthcare provider about this risk and the benefits of treating your asthma with ADVAIR DISKUS.
- ADVAIR DISKUS does not relieve sudden symptoms. Always have a short-acting beta2-agonist medicine with you to treat sudden symptoms. If you do not have an inhaled, short-acting bronchodilator, contact your healthcare provider to have one prescribed for you.
- Do not stop using ADVAIR DISKUS unless told to do so by your healthcare provider because your symptoms might get worse.
- ADVAIR DISKUS should be used only if your healthcare provider decides that another asthma-controller medicine alone does not control your asthma or that you need 2 asthma-controller medicines.
- Call your healthcare provider if breathing problems worsen over time while using ADVAIR DISKUS. You may need different treatment.
-
Get emergency medical care if:
- breathing problems worsen quickly, and
- you use your short-acting beta2-agonist medicine, but it does not relieve your breathing problems.
What is ADVAIR DISKUS?
ADVAIR DISKUS combines an inhaled corticosteroid medicine, fluticasone propionate (the same medicine found in FLOVENT) and a long-acting beta2 -agonist medicine, salmeterol (the same medicine found in SEREVENT). ADVAIR DISKUS is used for asthma and chronic obstructive pulmonary disease (COPD) as follows:
Asthma
ADVAIR DISKUS is used long term, twice a day to control symptoms of asthma and to prevent symptoms such as wheezing in adults and children ages 4 and older.
ADVAIR DISKUS contains salmeterol (the same medicine found in SEREVENT). Because LABA medicines, such as salmeterol, may increase the chance of death from asthma problems, ADVAIR DISKUS is not for adults and children with asthma who:
- are well controlled with another asthma-controller medicine such as a low to medium dose of an inhaled corticosteroid medicine
- only need short-acting beta2-agonist medicines once in awhile
Chronic Obstructive Pulmonary Disease
COPD is a chronic lung disease that includes chronic bronchitis, emphysema, or both. ADVAIR DISKUS 250/50 is used long term, twice a day to help improve lung function for better breathing in adults with COPD. ADVAIR DISKUS 250/50 has been shown to decrease the number of flare-ups and worsening of COPD symptoms (exacerbations).
Who should not use ADVAIR DISKUS?
Do not use ADVAIR DISKUS:
- to treat sudden, severe symptoms of asthma or COPD
- if you have a severe allergy to milk proteins. Ask your doctor if you are not sure.
What should I tell my healthcare provider before using ADVAIR DISKUS?
Tell your healthcare provider about all of your health conditions, including if you:
- have heart problems
- have high blood pressure
- have seizures
- have thyroid problems
- have diabetes
- have liver problems
- have osteoporosis
- have an immune system problem
- are pregnant or planning to become pregnant. It is not known if ADVAIR DISKUS may harm your unborn baby.
- are breastfeeding. It is not known if ADVAIR DISKUS passes into your milk and if it can harm your baby.
- are allergic to any of the ingredients in ADVAIR DISKUS, any other medicines, or food products. See the end of this Medication Guide for a complete list of the ingredients in ADVAIR DISKUS.
- are exposed to chickenpox or measles
Tell your healthcare provider about all the medicines you take including prescription and non-prescription medicines, vitamins, and herbal supplements. ADVAIR DISKUS and certain other medicines may interact with each other. This may cause serious side effects. Especially, tell your healthcare provider if you take ritonavir. The anti-HIV medicines NORVIR® (ritonavir capsules) Soft Gelatin, NORVIR (ritonavir oral solution), and KALETRA® (lopinavir/ritonavir) Tablets contain ritonavir.
Know the medicines you take. Keep a list and show it to your healthcare provider and pharmacist each time you get a new medicine.
How do I use ADVAIR DISKUS?
See the step-by-step instructions for using ADVAIR DISKUS at the end of this Medication Guide. Do not use ADVAIR DISKUS unless your healthcare provider has taught you and you understand everything. Ask your healthcare provider or pharmacist if you have any questions.
- Children should use ADVAIR DISKUS with an adult’s help, as instructed by the child’s healthcare provider.
- Use ADVAIR DISKUS exactly as prescribed. Do not use ADVAIR DISKUS more often than prescribed. ADVAIR DISKUS comes in 3 strengths. Your healthcare provider will prescribe the one that is best for your condition.
- The usual dosage of ADVAIR DISKUS is 1 inhalation twice a day (morning and evening). The 2 doses should be about 12 hours apart. Rinse your mouth with water after using ADVAIR DISKUS.
- If you take more ADVAIR DISKUS than your doctor has prescribed, get medical help right away if you have any unusual symptoms, such as worsening shortness of breath, chest pain, increased heart rate, or shakiness.
- If you miss a dose of ADVAIR DISKUS, just skip that dose. Take your next dose at your usual time. Do not take 2 doses at one time.
- Do not use a spacer device with ADVAIR DISKUS.
- Do not breathe into ADVAIR DISKUS.
- While you are using ADVAIR DISKUS twice a day, do not use other medicines that contain a long-acting beta2-agonist or LABA for any reason. Ask your healthcare provider or pharmacist if any of your other medicines are LABA medicines.
- Do not change or stop any of your medicines used to control or treat your breathing problems. Your healthcare provider will adjust your medicines as needed.
- Make sure you always have a short-acting beta2-agonist medicine with you. Use your short-acting beta2-agonist medicine if you have breathing problems between doses of ADVAIR DISKUS.
-
Call your healthcare provider or get medical care right away if:
- your breathing problems worsen with ADVAIR DISKUS
- you need to use your short-acting beta2-agonist medicine more often than usual
- your short-acting beta2-agonist medicine does not work as well for you at relieving symptoms
- you need to use 4 or more inhalations of your short-acting beta2-agonist medicine for 2 or more days in a row
- you use 1 whole canister of your short-acting beta2-agonist medicine in 8 weeks’ time
- your peak flow meter results decrease. Your healthcare provider will tell you the numbers that are right for you.
- you have asthma and your symptoms do not improve after using ADVAIR DISKUS regularly for 1 week
What are the possible side effects with ADVAIR DISKUS?
- ADVAIR DISKUS contains salmeterol (the same medicine found in SEREVENT). In patients with asthma, LABA medicines, such as salmeterol, may increase the chance of death from asthma problems. See “What is the most important information I should know about ADVAIR DISKUS?”
- Patients with COPD have a higher chance of getting pneumonia. ADVAIR DISKUS may increase the chance of getting pneumonia. Call your healthcare provider if you notice any of the following symptoms:
- increase in mucus (sputum) production
- change in mucus color
- fever
- chills
- increased cough
- increased breathing problems.
Other possible side effects with ADVAIR DISKUS include:
-
serious allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following symptoms of a serious allergic reaction, including:
- rash
- hives
- swelling of the face, mouth, and tongue
- breathing problems
- increased blood pressure
- a fast and irregular heartbeat
- chest pain
- headache
- tremor
- nervousness
- weakened immune system and a higher chance of infections
- lower bone mineral density. This may be a problem for people who already have a higher chance of low bone density (osteoporosis).
- eye problems including glaucoma and cataracts. You should have regular eye exams while using ADVAIR DISKUS.
- slowed growth in children. A child’s growth should be checked often.
The most common side effects with ADVAIR DISKUS include:
Asthma in adults and children:
- upper respiratory tract infection
- throat irritation
- hoarseness and voice changes
- thrush in the mouth and throat
- bronchitis
- cough
- headache
- nausea and vomiting
In children with asthma, infections in the ear, nose, and throat are also common.
COPD:
- thrush in the mouth and throat
- throat irritation
- hoarseness and voice changes
- viral respiratory infections
- headache
- muscle and bone pain
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the side effects with ADVAIR DISKUS. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store ADVAIR DISKUS?
- Store ADVAIR DISKUS at room temperature between 68° to 77° F (20° to 25° C). Keep in a dry place away from heat and sunlight.
- Safely discard ADVAIR DISKUS 1 month after you remove it from the foil pouch, or after the dose indicator reads “0”, whichever comes first.
- Keep ADVAIR DISKUS and all medicines out of the reach of children.
General Information about ADVAIR DISKUS
Medicines are sometimes prescribed for purposes not mentioned in a Medication Guide. Do not use ADVAIR DISKUS for a condition for which it was not prescribed. Do not give your ADVAIR DISKUS to other people, even if they have the same condition. It may harm them.
This Medication Guide summarizes the most important information about ADVAIR DISKUS. If you would like more information, talk with your healthcare provider or pharmacist. You can ask your healthcare provider or pharmacist for information about ADVAIR DISKUS that was written for healthcare professionals. You can also contact the company that makes ADVAIR DISKUS (toll free) at 1-888-825-5249 or at www.advair.com.
What are the ingredients in ADVAIR DISKUS?
Active ingredients: fluticasone propionate, salmeterol xinafoate
Inactive ingredient: lactose (contains milk proteins)
Instructions for Using ADVAIR DISKUS
Follow the instructions below for using your ADVAIR DISKUS. You will breathe in (inhale) the medicine from the DISKUS®. If you have any questions, ask your healthcare provider or pharmacist.
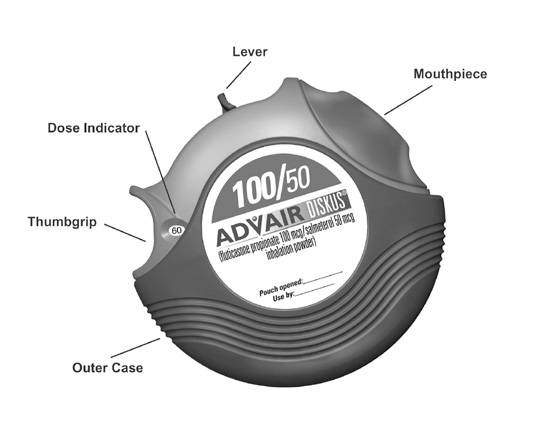
Take ADVAIR DISKUS out of the box and foil pouch. Write the “Pouch opened” and “Use by” dates on the label on top of the DISKUS. The “Use by” date is 1 month from date of opening the pouch.
- The DISKUS will be in the closed position when the pouch is opened.
- The dose indicator on the top of the DISKUS tells you how many doses are left. The dose indicator number will decrease each time you use the DISKUS. After you have used 55 doses from the DISKUS, the numbers 5 to 0 will appear in red to warn you that there are only a few doses left (see Figure 1). If you are using a “sample” DISKUS, the numbers 5 to 0 will appear in red after 9 doses.

Figure 1
Taking a dose from the DISKUS requires the following 3 simple steps: Open, Click, Inhale.
1. OPEN
Hold the DISKUS in one hand and put the thumb of your other hand on the thumbgrip. Push your thumb away from you as far as it will go until the mouthpiece appears and snaps into position (see Figure 2).

Figure 2
2. CLICK
Hold the DISKUS in a level, flat position with the mouthpiece towards you. Slide the lever away from you as far as it will go until it clicks(see Figure 3). The DISKUS is now ready to use.

Figure 3
Every time the lever is pushed back, a dose is ready to be inhaled. This is shown by a decrease in numbers on the dose counter. To avoid releasing or wasting doses once the DISKUS is ready:
- Do not close the DISKUS.
- Do not tilt the DISKUS.
- Do not play with the lever.
- Do not move the lever more than once.
3. INHALE
Before inhaling your dose from the DISKUS, breathe out (exhale) fully while holding the DISKUS level and away from your mouth (see Figure 4). Remember, never breathe out into the DISKUS mouthpiece.

Figure 4
Put the mouthpiece to your lips (see Figure 5). Breathe in quickly and deeply through the DISKUS. Do not breathe in through your nose.

Figure 5
Remove the DISKUS from your mouth. Hold your breath for about 10 seconds, or for as long as is comfortable. Breathe out slowly.
The DISKUS delivers your dose of medicine as a very fine powder. Most patients can taste or feel the powder. Do not use another dose from the DISKUS if you do not feel or taste the medicine.
Rinse your mouth with water after breathing-in the medicine. Spit the water out. Do not swallow.
4. Close the DISKUS when you are finished taking a dose so that the DISKUS will be ready for you to take your next dose. Put your thumb on the thumbgrip and slide the thumbgrip back towards you as far as it will go (see Figure 6). The DISKUS will click shut. The lever will automatically return to its original position. The DISKUS is now ready for you to take your next scheduled dose, due in about 12 hours. (Repeat steps 1 to 4.)

Figure 6
Remember:
- Never breathe into the DISKUS.
- Never take the DISKUS apart.
- Always ready and use the DISKUS in a level, flat position.
- Do not use the DISKUS with a spacer device.
- After each dose, rinse your mouth with water and spit the water out. Do not swallow.
- Never wash the mouthpiece or any part of the DISKUS. Keep it dry.
- Always keep the DISKUS in a dry place.
- Never take an extra dose, even if you did not taste or feel the medicine.
October 2008 ADD:5MG
This Medication Guide has been approved by the U.S. Food and Drug Administration.
ADVAIR DISKUS, DISKHALER, DISKUS, FLOVENT, ROTADISK, SEREVENT, and VENTOLIN are registered trademarks of GlaxoSmithKline.
NORVIR and KALETRA are registered trademarks of Abbott Laboratories.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2008, GlaxoSmithKline. All rights reserved.