MEDICATION GUIDE
Methylphenidate HCl Chewable Tablet, CII
(meth'' il fen' i date hye'' droe klor' ide)
Read the Medication Guide that comes with methylphenidate hydrochloride chewable tablets before you or your child starts taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your or your child’s treatment with methylphenidate hydrochloride chewable tablets.
|
What is the most important information I should know about methylphenidate hydrochloride chewable tablets? Methylphenidate hydrochloride chewable tablets may cause serious side effects, including:


 |
What Are methylphenidate hydrochloride chewable tablets?
Methylphenidate hydrochloride chewable tablets are a central nervous system stimulant prescription medicine. Methylphenidate hydrochloride chewable tablets are tablets that are made to be chewed and swallowed. They are used for the treatment of Attention Deficit and Hyperactivity Disorder (ADHD). Methylphenidate hydrochloride chewable tablets may help increase attention and decrease impulsiveness and hyperactivity in patients with ADHD.
Methylphenidate hydrochloride chewable tablets should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
Methylphenidate hydrochloride chewable tablets are also used in the treatment of a sleep disorder called narcolepsy.
|
Methylphenidate hydrochloride chewable tablets are a federally controlled substance (CII) because it contains methylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep methylphenidate hydrochloride chewable tablets in a safe place to protect it from theft. Never give your methylphenidate hydrochloride chewable tablets to anyone else, because it may cause death or harm them. Selling or giving away methylphenidate hydrochloride chewable tablets may harm others, and is against the law. |
Who should not take methylphenidate hydrochloride chewable tablets?
Methylphenidate hydrochloride chewable tablets should not be taken if you or your child:
- are very anxious, tense, or agitated
- have eye problems, including increased pressure in your eye, glaucoma, or problems with your close-up vision (farsightedness)
- have or had repeated movements or sounds (tics) or Tourette’s syndrome, or have a family history of tics or Tourette’s syndrome
- are taking or have taken within the past 14 days an antidepression medicine called a monoamine oxidase inhibitor or MAOI.
- are allergic to anything in methylphenidate hydrochloride chewable tablets. See the end of this Medication Guide for a complete list of ingredients.
Methylphenidate hydrochloride chewable tablets should not be used in children less than 6 years old because they have not been studied in this age group.
Methylphenidate hydrochloride chewable tablets may not be right for you or your child. Before starting methylphenidate hydrochloride chewable tablets tell your or your child’s healthcare provider about all health conditions (or a family history of) including:
- have heart problems, heart disease, heart defects, or high blood pressure
- mental problems including psychosis, mania, bipolar illness, or depression
- tics or Tourette’s syndrome
- seizures or have had an abnormal brain wave test (EEG)
- circulation problems in fingers and toes
Tell your healthcare provider if you or your child is pregnant, planning to become pregnant, or breastfeeding.
Can methylphenidate hydrochloride chewable tablets be taken with other medicines?
Tell your healthcare provider about all of the medicines that you or your child take including prescription and nonprescription medicines, vitamins, and herbal supplements.
Methylphenidate hydrochloride chewable tablets and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking methylphenidate hydrochloride chewable tablets.
Your healthcare provider will decide whether methylphenidate hydrochloride chewable tablets can be taken with other medicines.
Especially tell your healthcare provider if you or your child takes:
- antidepression medicines including MAOIs
- seizure medicines
- blood thinner medicines
- blood pressure medicines
- cold or allergy medicines that contain decongestants
Know the medicines that you or your child takes. Keep a list of your medicines with you to show your healthcare provider and pharmacist.
Do not start any new medicine while taking methylphenidate hydrochloride chewable tablets without talking to your healthcare provider first.
How should methylphenidate hydrochloride chewable tablets be taken?
- Take methylphenidate hydrochloride chewable tablets exactly as prescribed. Your healthcare provider may adjust the dose until it is right for you or your child.
- Methylphenidate hydrochloride chewable tablets are usually taken 2 to 3 times a day.
- Take methylphenidate hydrochloride chewable tablets 30 to 45 minutes before a meal.
|
- From time to time, your healthcare provider may stop methylphenidate hydrochloride chewable tablets treatment for awhile to check ADHD symptoms.
- Your healthcare provider may do regular checks of the blood, heart, and blood pressure while you are taking methylphenidate hydrochloride chewable tablets. Children should have their height and weight checked often while taking methylphenidate hydrochloride chewable tablets. Methylphenidate hydrochloride chewable tablets treatment may be stopped if a problem is found during these check-ups.
If you or your child take too much methylphenidate hydrochloride chewable tablets, call your healthcare provider or Poison Helpline, at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What are possible side effects of methylphenidate hydrochloride chewable tablets?
See “What is the most important information I should know about methylphenidate hydrochloride chewable tablets?” for information on reported heart and mental problems.
Other serious side effects include:
- Slowing of growth (height and weight) in children. Children should have their height and weight checked often during treatment with methylphenidate hydrochloride chewable tablets. Your healthcare provider may stop your child’s methylphenidate hydrochloride chewable tablets treatment if they are not growing or gaining weight as expected.
- Seizures, mainly in patients with a history of seizures
- Painful and prolonged erections (priapism) have occurred with methylphenidate. If you or your child develop priapism, seek medical help right away. Because of the potential for lasting damage, priapism should be evaluated by a healthcare provider immediately.
- Circulation problems in fingers and toes (peripheral vasculopathy, including Raynaud’s phenomenon): fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red. Tell your healthcare provider if you have or your child has numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes or if you have or your child has any signs of unexplained wounds appearing on fingers or toes while taking methylphenidate hydrochloride chewable tablets.
- Eyes problems (increased pressure in the eye and glaucoma). Call your healthcare provider right away if you or your child develop changes in your vision or eye pain, swelling, or redness.
- New or worsening tics or worsening Tourette’s syndrome. Tell your healthcare provider if you or your child get any new or worsening tics or worsening Tourette’s syndrome during treatment with methylphenidate hydrochloride chewable tablets.
Common side effects include:
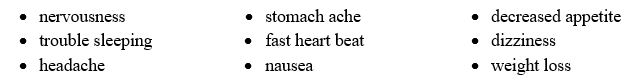
Talk to your healthcare provider if you or your child has side effects that are bothersome or do not go away.
This is not a complete list of possible side effects. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store methylphenidate hydrochloride chewable tablets?
- Store methylphenidate hydrochloride chewable tablets in a safe place at room temperature, at 20° to 25°C (68° to 77°F). [see USP Controlled Room Temperature]. Protect from moisture.
- Dispose of remaining, unused, or expired methylphenidate hydrochloride chewable tablets by a medicine take back program at a U.S. Drug Enforcement Administration (DEA) authorized collection site. If no takeback program or DEA authorized collector is available, mix methylphenidate hydrochloride chewable tablets with an undesirable, nontoxic substance such as dirt, cat litter, or used coffee grounds to make it less appealing to children and pets. Place the mixture in a container such as a sealed plastic bag and throw away methylphenidate hydrochloride chewable tablets in the household trash. Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
- Keep methylphenidate hydrochloride chewable tablets and all medicines out of the reach of children.
General information about methylphenidate hydrochloride chewable tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use methylphenidate hydrochloride chewable tablets for a condition for which it was not prescribed. Do not give methylphenidate hydrochloride chewable tablets to other people, even if they have the same condition. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about methylphenidate hydrochloride chewable tablets. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about methylphenidate hydrochloride chewable tablets that is written for healthcare professionals. For more information, please contact Camber Pharmaceuticals, Inc. at 1-866-495-8330.
What are the ingredients in methylphenidate hydrochloride chewable tablets?
|
CAUTION PHENYLKETONURICS: Methylphenidate hydrochloride chewable tablets contain phenylalanine. |
Active Ingredient: methylphenidate hydrochloride USP
Inactive Ingredients: aspartame, lactose anhydrous, microcrystalline cellulose, guar gum, grape flavor, pregelatinized starch, and stearic acid.
Medication Guide available at http://camberpharma.com/medication-guides
Manufactured by:
Ascent Pharmaceuticals, Inc.
Central Islip, NY 11722
Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised 09/2023
