MEDICATION GUIDE
Ibandronate Sodium (eye ban′ droe nate soe′ dee um)
Injection for Intravenous Use
Read the Medication Guide that comes with ibandronate sodium injection before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or treatment. Talk to your doctor if you have any questions about ibandronate sodium injection.
What is the most important information I should know about ibandronate sodium injection?
Ibandronate sodium injection is given in your vein (intravenously) and only given by a healthcare provider. Do not give ibandronate sodium injection to yourself.
Ibandronate sodium injection may cause serious side effects including:
- 1. Low calcium levels in your blood (hypocalcemia)
- 2. Severe allergic reaction (anaphylactic reaction)
- 3. Severe kidney problems
- 4. Severe jaw bone problems (osteonecrosis)
- 5. Bone, joint or muscle pain
- 6. Unusual thigh bone fractures
1. Low calcium levels in your blood (hypocalcemia).
Ibandronate sodium injection may lower the calcium levels in your blood. If you have low blood calcium before you start taking ibandronate sodium injection, it may get worse during treatment. Your low blood calcium must be treated before you receive ibandronate sodium injection. Most people with low blood calcium levels do not have symptoms, but some people may have symptoms. Call your doctor right away if you have symptoms of low blood calcium such as:
- •
- Spasms, twitches, or cramps in your muscles
- •
- Numbness or tingling in your fingers, toes, or around your mouth
Your doctor may prescribe calcium and vitamin D to help prevent low calcium levels in your blood, while you receive ibandronate sodium injection. Take calcium and vitamin D as your doctor tells you to.
2. Severe allergic reactions.
Some people who received ibandronate sodium injection had severe allergic reactions (anaphylactic reactions) that led to death. Get medical help right away if you have any of the symptoms of a serious allergic reaction such as:
- •
- Swelling of your face, lips, mouth or tongue
- •
- Trouble breathing
- •
- Wheezing
- •
- Severe itching
- •
- Skin rash, redness or swelling
- •
- Dizziness or fainting
- •
- Fast heartbeat or pounding in your chest
- •
- Sweating
3. Severe kidney problems.
Severe kidney problems, including kidney failure, may happen when you receive ibandronate sodium injection. Your doctor should do blood tests to check your kidneys before you receive each dose of ibandronate sodium injection.
4. Severe jaw bone problems (osteonecrosis).
Severe jaw bone problems may happen when you receive ibandronate sodium injection. Your doctor may examine your mouth before you start ibandronate sodium injection. Your doctor may tell you to see your dentist before you start ibandronate sodium injection. It is important for you to practice good mouth care during treatment with ibandronate sodium injection.
5. Bone, joint, or muscle pain.
Some people who receive ibandronate sodium injection develop severe bone, joint, or muscle pain.
6. Unusual thigh bone fractures.
Some people have developed unusual fractures in their thigh bone. Symptoms of a fracture may include new or unusual pain in your hip, groin, or thigh.
Call your doctor right away if you have any of these side effects.
What is ibandronate sodium injection?
Ibandronate sodium injection is a prescription medicine used to treat osteoporosis in women after menopause. Ibandronate sodium injection helps increase bone mass and helps reduce the chance of having a spinal fracture (break).
It is not known how long ibandronate sodium injection works for the treatment of osteoporosis. You should see your doctor regularly to determine if ibandronate sodium injection is still right for you.
It is not known if ibandronate sodium injection is safe and effective in children.
Who should not receive ibandronate sodium injection?
Do not receive ibandronate sodium injection if you:
- •
- Have low levels of calcium in your blood
- •
- Are allergic to ibandronate sodium or any of the ingredients in ibandronate sodium injection. See the end of this leaflet for a complete list of ingredients in ibandronate sodium injection.
What should I tell my healthcare provider before receiving ibandronate sodium injection?
Before you receive ibandronate sodium injection, tell your doctor if you:
- •
- Have low blood calcium
- •
- Plan to have dental surgery or teeth removed
- •
- Have kidney problems or other problems that may affect your kidneys
- •
- Have been told you have trouble absorbing minerals in your stomach or intestines (malabsorption syndrome)
- •
- Are pregnant or plan to become pregnant. It is not known if ibandronate sodium injection can harm your unborn baby.
- •
- Are breast-feeding or plan to breast-feed. It is not known if ibandronate sodium passes into your milk and may harm your baby.
Tell your doctor and dentist about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist each time you get a new medicine.
How should I receive ibandronate sodium injection?
- •
- Ibandronate sodium injection is given 1 time every 3 months by a healthcare provider.
- •
- If you miss a dose of ibandronate sodium injection, call your doctor or healthcare provider to schedule your next dose.
What are the possible side effects of ibandronate sodium injection?
Ibandronate sodium injection may cause serious side effects.
- •
- See “What is the most important information I should know about ibandronate sodium injection?”
The most common side effects of ibandronate sodium injection include:
- •
- Pain in your bones, joints or muscles
- •
- Back pain
- •
- Abdominal pain
- •
- Flu-like symptoms may happen within 3 days after you receive ibandronate sodium injection. Symptoms include:
- •
- fever
- •
- chills
- •
- bone, joint, or muscle pain
- •
- fatigue
If you have flu-like symptoms, they should get better within 24 to 48 hours.
Some people have pain or a sore that will not heal in their mouth or jaw while they receive ibandronate sodium injection. Tell your doctor or dentist if you have mouth or jaw problems.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of ibandronate sodium injection. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑ 800-FDA-1088.
You may also report side effects to Mylan Pharmaceuticals Inc. at 1-877-446-1088.
How should I store ibandronate sodium injection if I need to pick it up from a pharmacy?
- •
- Store ibandronate sodium injection at room temperature between 68°F and 77°F (20°C and 25°C).
Keep ibandronate sodium injection and all medicines out of the reach of children.
General information about the safe and effective use of ibandronate sodium injection.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ibandronate sodium injection for a condition for which it was not prescribed. Do not give ibandronate sodium injection to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about ibandronate sodium injection. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about ibandronate sodium injection that is written for health professionals.
What are the ingredients in ibandronate sodium injection?
Active ingredient: ibandronate sodium.
Inactive ingredients: sodium chloride, glacial acetic acid, sodium acetate and water.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
For more information, call Mylan Pharmaceuticals Inc at 1-877-446-3679 (1-877-4-INFO-RX).
Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.
Manufactured by:
Mylan Laboratories Limited
Bangalore, India
JANUARY 2018
TERUMO® SurshieldTM safety Winged Infusion Set-
Instructions for Use: IV Administration
Aseptic technique, proper skin preparation and continued protection of the site are essential. Observe Universal Precautions on all patients.
Caution: Keep hands behind the needle at all times during use and disposal.
Instructions for Device Assembly
- •
- Open the package.
- •
- Remove the rubber cap from the tip of the syringe containing the ibandronate injection and the protective SV cap from hub at the end of the tubing opposite the butterfly needle.
- •
- Insert the tip of the syringe into the hub and twist as firm pressure is applied to assure a tight connection.
- •
- Proceed with priming and confirm that administration fluid comes from the needle.
Venipuncture and Administration
- •
- Flip the safety shield back away from the needle towards the tubing. Grasp wings securely.
- •
- Remove the needle protector. Caution: Care should be taken not to touch the needle.
- •
- Perform venipuncture and confirm proper positioning of the needle in the vein.
- •
- Carefully allow wings to return to starting position and conform to the shape of the skin.
- •
- Further secure the position of the winged infusion set per facility protocol.
After Use
- •
- Remove tape, if present, from wings.
- •
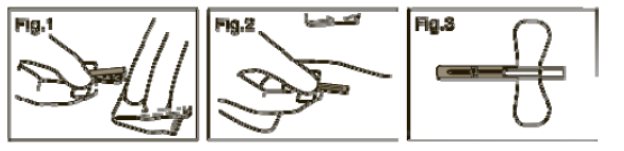
- Flip the safety shield forward toward the needle. Grasp a wing and the safety shield between your thumb and index finger. Completely remove the needle from the puncture site and apply digital pressure to the site using a sterile gauze pad held in the opposite hand (Fig. 1).
- •
- With the wing and shield between your thumb and index finger pinch together (or press the safety shield against a hard surface such as a bedside table) until an audible click is heard (Fig. 2).
- •
- Visually confirm activation of the safety feature (Fig. 3).
- •
- Dispose of used needles and materials following the policies and procedures of your facility, as well as federal and local regulations for “Sharps Disposal.”
®Registered Trademark. Surshield is a trademark of TERUMO CORPORATION.