INSTRUCTIONS FOR USE
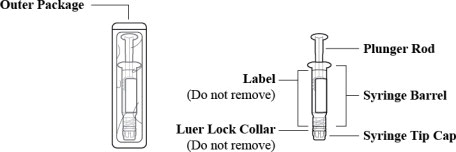
Figure 1: Outer Packaging and Prefilled Syringe
NOTES:
- - Do not introduce any other fluid into the syringe at any time.
- - Do not dilute for IV push.
- - Do not re-sterilize the syringe.
- - Do not use this product on a sterile field.
- - This product is for single dose only.
- Inspect the outer packaging (blister pack) to confirm the integrity of the packaging. Do not use if the blister pack or the prefilled syringe has been damaged.
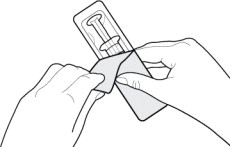
- Remove the syringe from the outer packaging. (See Figure 2)
- Visually inspect the syringe. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
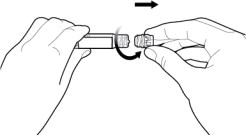
- Twist off the syringe tip cap. Do not remove the label around the luer lock collar. (See Figure 3)
- Expel air bubble(s). Adjust the dose (if applicable).
- Administer the dose ensuring that pressure is maintained on the plunger rod during the entire administration.
- Discard the used syringe into an appropriate receptacle.
For more information concerning this drug, please call Fresenius Kabi USA, LLC at 1-800-551-7176.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
The brand names mentioned in this document are the trademarks of their respective owners.
U.S. Patent 9,731,082
www.fresenius-kabi.com/us
451612
Revised: 10/2020
Revised: 1/2021
Document Id: 916ef348-1e5b-40b4-9d12-508fc2ce567c
Set id: 88d25d19-acff-477b-86c8-9797782045b8
Version: 13
Effective Time: 20210120
Fresenius Kabi USA, LLC