FREAMINE HBC- isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, alanine, arginine, histidine, proline, serine, glycine, cysteine, and sodium bisulfite injection, solution
B. Braun Medical Inc.
----------
Directions for Use of B. Braun Glass Containers with Solid Stoppers
Designed for use with a vented set. Use 18 to 22 gauge needle size for admixing or withdrawing solutions from the glass bottle.
Before use, perform the following checks:
- Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
- Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter; check the bottle for cracks or other damage. In checking for cracks, do not be confused by normal surface marks and seams on bottom and sides of bottle. These are not flaws. Look for bright reflections that have depth and penetrate into the wall of the bottle. Reject any such bottle.
- To remove the outer closure, lift the tear tab and pull up, over, and down until it is below the stopper (see Figure 1). Use a circular pulling motion on the tab until it breaks away.
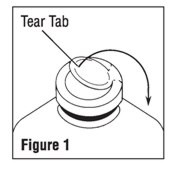
- Grasp and remove the metal disk, exercising caution not to touch the exposed sterile stopper surface.
Warning: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. - When adding medication to the container prior to administration, swab the target area of the rubber stopper, inject medication and mix thoroughly by gentle agitation.
- Refer to Directions for Use of the set being used. Insert the set spike into the bottle through the target area of the rubber stopper. Allow the fluid to flow and remove air from the tubing before administration begins. Hang the container.
- After admixture and during administration, re-inspect the solution frequently. If any evidence of solution contamination or instability is found or if the patient exhibits any signs of fever, chills or other reactions not readily explainable, discontinue administration immediately and notify the physician.
- Spiking, additions, or transfers should be made immediately after exposing the sterile stopper surface. Check for vacuum at first puncture of stopper. Admixture by needle or syringe should be made through the target area of the rubber stopper; contents should be drawn by vacuum into the bottle. Admixture by spiked vial should also be through the target area of the rubber stopper (see Figure 2). If contents of initial addition are not drawn into the bottle, vacuum is not present and the unit should be discarded. Each addition/transfer will reduce the vacuum remaining in the bottle.
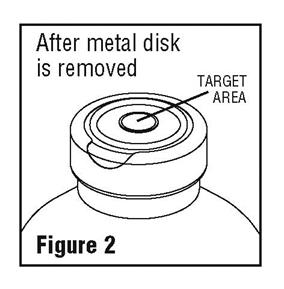
- If the first puncture of the stopper is the administration set spike, insert the spike fully into the target area of the rubber stopper and promptly invert the bottle. Verify vacuum by observing rising air bubbles. Do not use the bottle if vacuum is not present.
- If admixture or set insertion is not performed immediately following removal of protective metal disk, swab stopper surface.
CAUTION: Large partial fill containers (one liter or larger) have high vacuum to facilitate large additions or transfers. This creates an implosion hazard and requires special care in use and handling.
Revised: 3/2020
Document Id: 3b4cdd6b-0d48-4fa3-b36f-d4e670d99f8a
Set id: 6ea7ec73-403e-4303-8862-6380e2f1fda6
Version: 9
Effective Time: 20200331
B. Braun Medical Inc.