| MEDICATION GUIDE
Divalproex Sodium (dye val’ proe ex soe’ dee um) Delayed-release Capsules USP, for oral use |
| What is the most important information I should know about divalproex sodium delayed-release capsules?
Do not stop divalproex sodium delayed-release capsules without first talking to a healthcare provider. Stopping divalproex sodium delayed-release capsules suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).Divalproex sodium delayed-release capsules can cause serious side effects, including: 1.Serious liver damage that can cause death, especially in children younger than 2 years old and patients with mitochondrial disorders. The risk of getting this serious liver damage is more likely to happen within the first 6 months of treatment. Call your healthcare provider right away if you get any of the following symptoms:
2. Divalproex sodium delayed-release capsules may harm your unbornbaby.
Call your healthcare provider right away if you have any of these symptoms:
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
|
How can I watch for early symptoms of suicidal thoughts and actions?
Call your healthcare provider between visits as needed, especially if you are worried aboutsymptoms. Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. |
| What are divalproex sodium delayed-release capsules?
Divalproex sodium delayed-release capsules are prescription medicine used:
|
Do not take divalproex sodium delayed-release capsules if you:
|
Before taking divalproex sodium delayed-release capsules, tell your healthcare provider about all of your medical conditions including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Divalproex sodium delayed-release capsules may affect the way other medicines work, and other medicines may affect how divalproex sodium delayed-release capsules works. Using divalproex sodium delayed-release capsules with other medicines can cause serious side effects. Do not start or stop other medicines without talking to your healthcare provider. Especially tell your healthcare provider if you take:
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist each time you get a new medicine. |
How should I take divalproex sodium delayed-release capsules?
|
What should I avoid while taking divalproex sodium delayed-release capsules?
|
| What are the possible side effects of divalproex sodium delayed-release capsules?
Call your healthcare provider right away if you have any of the symptoms listed below. Your healthcare provider may do additional tests before and during your treatment with divalproex sodium delayed-release capsules. Your healthcare provider may reduce your dose, temporarily stop, or permanently stop treatment if you have certain side effects. |
Divalproex sodium delayed-release capsules can cause serious side effectsincluding:
• headache • loss of appetite • weakness • weight loss • sleepiness • increased appetite • dizziness • weight gain • tremors • nausea / vomiting • difficulty walking or problems with coordination • stomach pain • ringing in your ears • diarrhea • blurred vision • constipation • double vision • bronchitis • unusual eye movement • flu-like symptoms • hair loss (alopecia) • infection • swelling of your arms or legs These are not all of the possible side effects of divalproex sodium delayed-release capsules. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA- 1088. |
How should I store divalproex sodium delayed-release capsules?
|
| General information about the safe and effective use of divalproex sodium delayed-release capsules.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use divalproex sodium delayed-release capsules for a condition for which it was not prescribed. Do not give divalproex sodium delayed-release capsules to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about divalproex sodium delayed-release capsules that is written for health professionals. |
| What are the ingredients in divalproex sodium extended-release tablets?
Active ingredient: divalproex sodium Inactive ingredients: Divalproex sodium delayed-release capsules: black iron oxide, D&C Red 28, ethyl cellulose, FD&C Blue 1, gelatin, hypromellose, magnesium stearate, sugar sphere, titanium dioxide, and triethyl citrate NF. Distributed by:Dr. Reddy’s Laboratories Inc., Princeton, NJ 08540 Made in India For more information, call 1-888-375-3784. |
This Medication Guide has been approved by the U.S. Food and Drug Administration.
To reorder additional Medication Guides, contact Dr. Reddy’s Customer Service at 1-866-733-3952.
Revised: 03/2023
Read the Medication Guide and this Instructions for Use that come with divalproex sodium delayed-release capsules for the most important information you need to know before taking divalproex sodium delayed-release capsules for the first time, and each time you get a refill. This information does not take the place of talking to your healthcare provider about your medical treatment or condition.
Important information Take divalproex sodium delayed-release capsules exactly as your healthcare provider tells you. Your healthcare provider will tell you how much divalproex sodium delayed-release capsules to take and when to take it.
- Do not change your dose of divalproex sodium delayed-release capsules without talking to your healthcare provider.
- Divalproex sodium delayed-release capsules may be swallowed whole or the divalproex sodium delayed-release capsules may be opened and the contents mixed into soft food such as applesauce or pudding. The sprinkles are flavorless.
- Immediately take the medicine after mixing it with soft food. Do not chew the divalproex sodium delayed-release capsules and food mixture. Do not store the mixture for future use. Mix the divalproex sodium delayed-release capsules with soft food each time, right before it is taken.
- If you have any questions, contact your healthcare provider or pharmacist. Keep all of your healthcare provider's appointments as scheduled.s:
Supplies needed:
- Divalproex Sodium Delayed-release Capsule
- soft food such as applesauce or pudding
- teaspoon small cup or bowl
- water
Preparing and taking divalproex sodium delayed-release capsules with soft foods:
| 1 | Place a teaspoon of soft food into a small cup or bowl. | |
| 2 |  | Hold the divalproex sodium delayed-release capsule so that the end marked "This end up" is upright and the arrow on the capsule points up. The divalproex sodium delayed-release capsule is extra-large to help prevent spilling the contents, but handle it carefully. |
| 3 | 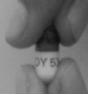 | Hold the divalproex sodium delayed-release capsule carefully over the cup or bowl of soft food. Gently twist the top of the capsule to separate the top from the bottom. If any of the divalproex sodium delayed-release capsule contents spills outside the cup or bowl, start over with a new capsule and a new cup or bowl of soft food. |
| 4 |  | Empty the divalproex sodium delayed-release capsule contents into the soft food, and stir it into the soft food with the teaspoon. |
| 5 |  | Swallow the divalproex sodium delayed-release capsule and food mixture right away. Do not chew it. Drinking water right after taking divalproex sodium delayed-release capsule and food mixture will help make sure all of the divalproex sodium delayed-release capsule’s sprinkles are swallowed. |
Storing divalproex sodium delayed-release capsules
• Store divalproex sodium delayed-release capsules below 20°-25°C (68°-77°F).
Keep divalproex sodium delayed-release capsules and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S Foodand Drug Administration.
Distributed by:
Dr. Reddy’s Laboratories Inc.,
Princeton, NJ 08540
Made in India
Revised: 03/2023