HEPATAMINE- isoleucine, leucine, lysine acetate, methionine, phenylalanine, threonine, tryptophan, valine, alani injection, solution
B. Braun Medical Inc.
----------
Directions for Use of B. Braun Glass Containers with Solid Stoppers
Designed for use with a vented set. Use 18 to 22 gauge needle size for admixing or withdrawing solutions from the glass bottle.
- Before use, perform the following checks:
- Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
- Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter; check the bottle for cracks or other damage. In checking for cracks, do not be confused by normal surface marks and seams on the bottom and sides of the bottle. These are not flaws. Look for bright reflections that have depth and penetrate into the wall of the bottle. Reject any such bottle.
2. Remove plastic cap (See Figure 1).
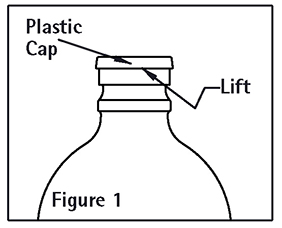
3. Swab exposed stopper surface with a suitable disinfectant.
Warning: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
4. When adding medication to the container prior to administration, swab the target area of the rubber stopper, inject medication and mix thoroughly by gentle agitation.
5. Refer to Directions for Use of the set being used. Insert the set spike into the bottle through the target area of the rubber stopper. Allow the fluid to flow and remove air from the tubing before administration begins. Hang the container.
6. After admixture and during administration, re-inspect the solution frequently. If any evidence of solution contamination or instability is found or if the patient exhibits any signs of fever, chills or other reactions not readily explainable, discontinue administration immediately and notify the physician.
7. Spiking, additions, or transfers should be made immediately after swabbing stopper surface. Check for vacuum at first puncture of stopper. Admixture by needle or syringe should be made through the target area of the rubber stopper; contents should be drawn by vacuum into the bottle. Admixture by spiked vial should be through the target area of the rubber stopper (See Figure 2). If contents of initial addition are not drawn into the bottle, vacuum is not present and the unit should be discarded. Each addition/transfer will reduce the vacuum remaining in the bottle.
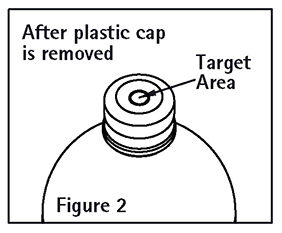
8. If the first puncture of the stopper is the administration set spike, insert the spike fully into the target area of the rubber stopper and promptly invert the bottle. Verify vacuum by observing rising air bubbles. Do not use the bottle if vacuum is not present.
9. If admixture or set insertion is not performed immediately following swabbing, swab stopper surface again with a suitable disinfectant.