FENTANYL- fentanyl patch
Mylan Pharmaceuticals Inc.
----------
Medication Guide
|
Fentanyl Transdermal System, CII
|
|
|
Fentanyl transdermal system is:
|
|
|
Important information about fentanyl transdermal system:
|
|
|
Do not use fentanyl transdermal system if you have:
|
|
|
Before applying fentanyl transdermal system, tell your healthcare provider if you have a history of: |
|
|
|
|
|
|
Tell your healthcare provider if you:
|
|
|
When using fentanyl transdermal system:
|
|
|
While using fentanyl transdermal system DO NOT:
|
|
|
The possible side effects of fentanyl transdermal system are:
Get emergency medical help or call 911 right away if you have:
These are not all the possible side effects of fentanyl transdermal system. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. For more information go to dailymed.nlm.nih.gov
Manufactured for: Mylan Pharmaceuticals Inc., Morgantown, WV 26505 U.S.A. For more information, call Mylan at 1-877-446-3679 (1-877-4-INFO-RX). |
|
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Instructions for Use
Fentanyl Transdermal System CII
(fen' ta nil)
Be sure that you read, understand, and follow these Instructions for Use before you apply fentanyl transdermal system (patch). Talk to your healthcare provider or pharmacist if you have any questions.
Important information about the fentanyl transdermal system (patch) appearance:
- •
- Fentanyl transdermal system is a rectangular, translucent patch with rounded corners.
- •
- Fentanyl transdermal system comes in 8 different dosage strengths and sizes:
|
|
- •
- The product’s active ingredient name, “Fentanyl”, and dosage strength are randomly printed in blue on each patch.
- •
- All of the patches have blue diagonal stripes.
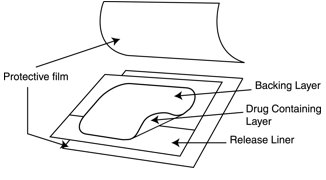
Parts of the fentanyl transdermal system:
BEFORE APPLYING FENTANYL TRANSDERMAL SYSTEM
- •
- Each fentanyl transdermal system is sealed in its own protective pouch. Do not remove a fentanyl transdermal system from the pouch until you are ready to use it.
- •
- Do not use a fentanyl transdermal system if the pouch seal is broken or the patch is cut, damaged or changed in any way.
- •
- Fentanyl transdermal systems are available in 8 different dosage strengths and patch sizes. Make sure you have the right dose patch or patches that have been prescribed for you.
APPLYING A FENTANYL TRANSDERMAL SYSTEM
- 1.
-
Skin areas where the fentanyl transdermal system may be applied:
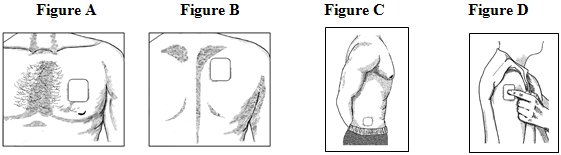
For adults: - •
- Put the patch on the chest, back, flank (sides of the waist), or upper arm in a place where there is no hair (See Figures A-D).
For children (and adults with mental impairment):
- •
- Put the patch on the upper back (See Figure B). This will lower the chances that the child will remove the patch and put it in their mouth.
For adults and children
- •
- Do not put a fentanyl transdermal system on skin that is very oily, burned, broken out, cut, irritated, or damaged in any way.
- •
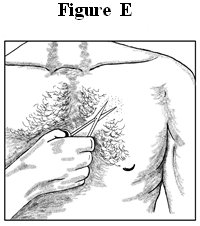
- Avoid sensitive areas or those that move around a lot. If there is hair, do not shave (shaving irritates the skin). Instead, clip hair as close to the skin as possible (See Figure E).
- •
-
Talk to your healthcare provider if you have questions about skin application sites.
- 2.
-
Prepare to apply a fentanyl transdermal system:
- •
- Choose the time of day that is best for you to apply fentanyl transdermal system. Change it at about the same time of day (3 days or 72 hours after you apply the patch) or as directed by your healthcare provider.
- •
- Do not wear more than one fentanyl transdermal system at a time unless your healthcare provider tells you to do so. Before applying a new fentanyl transdermal system, remove the patch you have been wearing.
- •
- Clean the skin area with clear water only. Pat skin completely dry. Do not use anything on the skin such as soaps, lotions, oils, or alcohol before the patch is applied.
- 3.
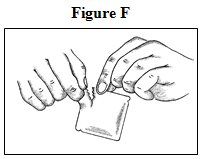
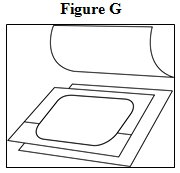
- Open the pouch: Tear at notch and remove the fentanyl transdermal system. Each fentanyl transdermal system is packaged with additional pieces of protective film above and below the patch and is sealed in its own protective pouch. Do not remove the fentanyl transdermal system from the pouch until you are ready to use it (See Figure F). The additional pieces of protective film are discarded at time of use (See Figure G).
- 4.
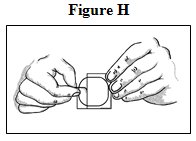
- Peel: Peel off both parts of the release liner from the patch. Each fentanyl transdermal system has a clear plastic release liner that can be peeled off in two pieces. This covers the sticky side of the patch. Carefully peel this release liner off and throw the pieces away. Touch the sticky side of the fentanyl transdermal system as little as possible (See Figure H).
- 5.
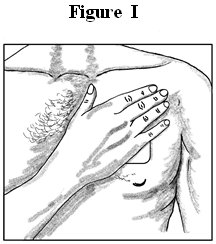
- Press: Press the patch onto the chosen skin site with the palm of your hand and hold there for at least 30 seconds (See Figure I). Make sure it sticks well, especially at the edges.
- •
- Fentanyl transdermal system may not stick to all people. You need to check the patch often to make sure that it is sticking well to the skin.
- •
- If the patch falls off right away after applying, throw it away and put a new one on at a different skin site. See the section below called “Disposing of a fentanyl transdermal system”.
- •
- If you have a problem with the patch not sticking
- o
- Apply first aid tape only to the edges of the patch.
- o
- If you continue to have problems with the patch not sticking, you may cover the patch with a transparent adhesive film dressing such as BIOCLUSIVE® or Askina®Derm. These are special see-through adhesive dressings. Never cover a fentanyl transdermal system with any other bandage or tape. Remove the liner from the BIOCLUSIVE® or Askina®Derm dressing and place it carefully over the fentanyl transdermal system, smoothing it over the patch and your skin.
- •
- If your patch falls off before 3 days (72 hours) of use, dispose of (throw away) properly. See the section below “Disposing of a fentanyl transdermal system”. Apply a new fentanyl transdermal system on at a different skin site. Be sure to let your healthcare provider know that this has happened, and do not replace the new patch until 3 days (72 hours) after you put it on (or as directed by your healthcare provider).
- 6.
- Wash your hands when you have finished applying a fentanyl transdermal system.
- 7.
- Remove a fentanyl transdermal system after wearing it for 3 days (72 hours). Dispose of the used patch right away. See the section below “Disposing of a fentanyl transdermal system”. Choose a different skin site to apply a new fentanyl transdermal system. Repeat Steps 2 through 6 above when applying a new fentanyl transdermal system.
Do not apply the new patch to the same place as the last one.
WATER AND FENTANYL TRANSDERMAL SYSTEM
- •
- You can bathe, swim or shower while you are wearing a fentanyl transdermal system. If the patch falls off before 3 days (72 hours) after application, dispose of properly. See the section below “Disposing of a fentanyl transdermal system”. Apply a new fentanyl transdermal system on at a different skin site. Be sure to let your healthcare provider know that this has happened, and do not replace the new patch until 3 days (72 hours) after you put it on (or as directed by your healthcare provider).
DISPOSING OF A FENTANYL TRANSDERMAL SYSTEM
- •
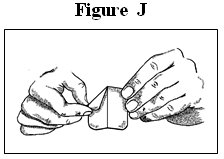
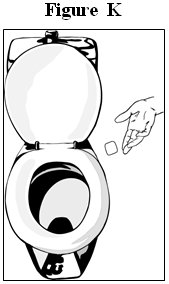
- Fold the used fentanyl transdermal system in half so that the sticky side sticks to itself (See Figure J). Flush the used fentanyl transdermal system down the toilet right away (See Figure K). A used fentanyl transdermal system can be very dangerous for or lead to death in babies, children, pets, and adults who have not been prescribed fentanyl transdermal system.
- •
- Throw away any fentanyl transdermal systems that are left over from your prescription as soon as they are no longer needed. Remove the leftover patches from their protective pouch and remove the release liner. Fold the patches in half with the sticky sides together, and flush the patches down the toilet. Do not flush the pouch, protective films or the release liner down the toilet. These items can be thrown away in a trash can.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
The brands listed are trademarks of their respective owners.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Revised: 12/2023
FTS:R31X