|
Hydrocodone Polistirex and
Chlorpheniramine Polistirex
Extended-Release Suspension, C-II
(hye” droe koe’ done pol” i stye’ rex and
klor” fen ir’ a mean pol” i stye’ rex)
|
|
What is the most important information I should know about Hydrocodone Polistirex and Chlorpheniramine Polistirex?
Hydrocodone Polistirex and Chlorpheniramine Polistirex is not for children under 18 years of age.
Hydrocodone Polistirex and Chlorpheniramine Polistirex can cause serious side effects, including:
-
Addiction, abuse and misuse. Taking Hydrocodone Polistirex and Chlorpheniramine Polistirex or other medicines that contain an opioid can cause addiction, abuse and misuse, which can lead to overdose and death. This can happen even if you take Hydrocodone Polistirex and Chlorpheniramine Polistirex exactly as prescribed by your healthcare provider. Your risk of addiction, abuse, and misuse is increased if you or a family member has a history of drug or alcohol abuse or addiction, or mental health problems.
-
Life-threatening breathing problems (respiratory depression). Hydrocodone Polistirex and Chlorpheniramine Polistirex can cause breathing problems (respiratory depression) that can happen at any time during treatment and can lead to death. Your risk of breathing problems is greatest when you first start taking Hydrocodone Polistirex and Chlorpheniramine Polistirex, are taking other medicines that can cause breathing problems, have certain lung problems, are elderly, or have certain other health problems.
Children are at higher risk for respiratory depression. Breathing problems can happen even if you take Hydrocodone Polistirex and Chlorpheniramine Polistirex exactly as prescribed by your healthcare provider.
Call your healthcare provider or get emergency medical help right away if anyone taking Hydrocodone Polistirex and Chlorpheniramine Polistirex
has any of the symptoms below:
-
confusion
-
difficulty breathing
-
shallow breathing
-
limpness
Keep Hydrocodone Polistirex and Chlorpheniramine Polistirex in a safe place away from children. Accidental use of even 1 dose of Hydrocodone Polistirex and Chlorpheniramine Polistirex, especially by a child, is a medical emergency and can cause breathing problems (respiratory depression) which can lead to death. If a child accidentally takes Hydrocodone Polistirex and Chlorpheniramine Polistirex, get emergency medical help right away.
-
Overdose and death due to medicine dosing errors. Overdose and death can happen if you measure the wrong dose of Hydrocodone Polistirex and Chlorpheniramine Polistirex. Always use an accurate milliliter measuring device to measure the correct amount of Hydrocodone Polistirex and Chlorpheniramine Polistirex. Do not use a household teaspoon to measure your medicine. You may accidentally take too much. You can ask your pharmacist for the measuring device you should use and how to measure the correct dose.
-
Breathing problems (respiratory depression) that can lead to death and opioid withdrawal can happen if you start taking or stop taking other medicines while taking Hydrocodone Polistirex and Chlorpheniramine Polistirex, including:
-
certain antibiotics
-
certain medicines to treat a fungal infection
-
certain medicines to treat Human Immunodeficiency Virus (HIV)-1 infection, Acquired Immune Deficiency
-
Syndrome (AIDS), or Hepatitis C
-
rifampin
|
|
What is Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Suspension?
-
Hydrocodone Polistirex and Chlorpheniramine Polistirex is a prescription medicine used in adults to treat cough and upper respiratory symptoms that you can have with allergies or a common cold. Hydrocodone Polistirex and Chlorpheniramine Polistirex contains 2 medicines, hydrocodone and chlorpheniramine. Hydrocodone is an opioid (narcotic) cough suppressant. Chlorpheniramine is an antihistamine.
-
Hydrocodone Polistirex and Chlorpheniramine Polistirex is a federal controlled substance (CII) because it contains hydrocodone that can be abused or lead to dependence. Keep Hydrocodone Polistirex and Chlorpheniramine Polistirex in a safe place to prevent misuse and abuse. Selling or giving away Hydrocodone Polistirex and Chlorpheniramine Polistirex may harm others, and is against the law. Tell your healthcare provider if you have abused or been dependent on alcohol, prescription medicines or street drugs.
|
|
Who should not take Hydrocodone Polistirex and Chlorpheniramine Polistirex?
Hydrocodone Polistirex and Chlorpheniramine Polistirex is not for children under 18 years of age. See “What is the most important information I should know about Hydrocodone Polistirex and Chlorpheniramine Polistirex?”
Do not take Hydrocodone Polistirex and Chlorpheniramine Polistirex if you:
-
have severe breathing problems (respiratory depression) or breathing problems caused by asthma. See “What is the most important information I should know about Hydrocodone Polistirex and Chlorpheniramine Polistirex?”
-
have a blockage (obstruction) in your bowel such as a paralytic ileus.
-
are allergic to hydrocodone, chlorpheniramine, or any of the ingredients in Hydrocodone Polistirex and Chlorpheniramine Polistirex. See the end of this Medication Guide for a complete list of ingredients.
Ask your healthcare provider if you have any questions about this information.
|
|
Before you take Hydrocodone Polistirex and Chlorpheniramine Polistirex, tell your healthcare provider about all of your medical conditions, including if you:.
-
have pain in your stomach-area (abdomen)
-
have constipation or bowel problem
-
have bile duct or pancreas problems
-
have prostate problems
-
have problems with your urinary tract or difficulty urinating
-
are pregnant or plan to become pregnant. Hydrocodone Polistirex and Chlorpheniramine Polistirex can harm your unborn baby. See “What is the most important information I should know about Hydrocodone Polistirex and Chlorpheniramine Polistirex?”
-
are breastfeeding or plan to breastfeed. Hydrocodone and chlorpheniramine pass into your breast milk and can cause serious side effects in your baby including increased sleepiness, breathing problems (respiratory depression), and death. You and your healthcare provider should decide if you will take Hydrocodone Polistirex and Chlorpheniramine Polistirex or breastfeed. You should not do both. See “What should I avoid while taking Hydrocodone Polistirex and Chlorpheniramine Polistirex?”
-
plan to have children. Hydrocodone Polistirex and Chlorpheniramine Polistirex may affect the ability to have a child in females and males (fertilityproblems). It is not known if these fertility problems will be reversible, even after you stop taking Hydrocodone Polistirex and Chlorpheniramine Polistirex. Talk to your healthcare provider if this is a concern for you.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking Hydrocodone Polistirex and Chlorpheniramine Polistirex with certain other medicines can cause side effects or affect how well Hydrocodone Polistirex and Chlorpheniramine Polistirex or the other medicines work. Do not start or stop taking other medicines without talking to your healthcare provider.
Especially tell your healthcare provider if you:
-
See “What should I avoid while taking Hydrocodone Polistirex and Chlorpheniramine Polistirex?”
-
take pain medicines such as opioids (narcotics).
-
take cold or allergy medicines that contain antihistamines or cough suppressants.
-
drink alcohol.
-
take muscle relaxants.
-
take certain medicines used to treat mood, anxiety, psychotic or thought disorders, or depression, including monoamine oxidase inhibitors (MAOIs), tricyclics, selective serotonin reuptake inhibitors (SSRIs), selective serotonin nnorepinephrine reuptake inhibitors (SNRIs), or antipsychotics.
-
take medicines to lower your blood pressure.
-
take water pills (diuretics).
-
take medicines called “anticholinergics” used to treat health problems such as asthma, chronic obstructive pulmonarydisease (COPD), or stomach problems.
-
take a medicine called “phenytoin” used to treat seizures or epilepsy.
Ask your healthcare provider if you are not sure if you take any of these medicines.
|
|
How should I take Hydrocodone Polistirex and Chlorpheniramine Polistirex?
-
See “What is the most important information I should know about Hydrocodone Polistirex and Chlorpheniramine Polistirex?”
-
Take Hydrocodone Polistirex and Chlorpheniramine Polistirex exactly as your healthcare provider tells you to take it. Do not change your dosewithout talking to your healthcare provider.
-
Take Hydrocodone Polistirex and Chlorpheniramine Polistirex by mouth only.
-
Take Hydrocodone Polistirex and Chlorpheniramine Polistirex using an accurate milliliter measuring device. . Only measure Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Suspension with the dosing cup that comes with your prescription. If you do not have a device, ask your pharmacist to give you a measuring device to help you measure the correct amount of Hydrocodone Polistirex and Chlorpheniramine Polistirex. Do not use a household teaspoon to measure your medicine. You may accidentally take too much.
-
The dosing cup is calibrated for measuring 2.5 mL and 5 mL dosages. Find the marked line for the dose you are taking.
-
Fill the cup so the medicine is leveled to the dose that has been prescribed. Do not fill over the dosage line.
-
Do not overfill the measuring device.
-
Rinse the measuring device with water after each use.
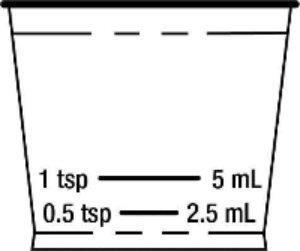 Cup
-
If you take too much Hydrocodone Polistirex and Chlorpheniramine Polistirex, call your healthcare provider or go to the nearest hospital emergency room right away.
-
Tell your healthcare provider if your cough does not get better within 5 days of treatment with Hydrocodone Polistirex and Chlorpheniramine Polistirex.
|
|
What should I avoid while taking Hydrocodone Polistirex and Chlorpheniramine Polistirex?
-
Avoid driving a car or operating machinery during treatment with Hydrocodone Polistirex and Chlorpheniramine Polistirex. Hydrocodone Polistirex and Chlorpheniramine Polistirex can cause you to be drowsy, slow your thinking and motor skills, and may affect your vision.
-
Do not drink alcohol during treatment with Hydrocodone Polistirex and Chlorpheniramine Polistirex. Drinking alcohol can increase your chances of having serious side effects.
Avoid the use of Hydrocodone Polistirex and Chlorpheniramine Polistirex if you:
-
are pregnant. Use of Hydrocodone Polistirex and Chlorpheniramine Polistirex during pregnancy can cause withdrawal symptoms in your newborn baby that could be life-threatening if not recognized and treated. Tell your healthcare provider right away if you are pregnant or think you may be pregnant.
-
are breastfeeding. Use of Hydrocodone Polistirex and Chlorpheniramine Polistirex while breastfeeding can cause severe breathing problems (respiratory depression) in your breastfed infant that could be life-threatening.
-
take a medicine called a monoamine oxidase inhibitor (MAOI). Avoid taking an MAOI within 14 days after you stop taking Hydrocodone Polistirex and Chlorpheniramine Polistirex. Avoid starting Hydrocodone Polistirex and Chlorpheniramine Polistirex if you stopped taking an MAOI in the last 14 days.
|
What are the possible side effects of Hydrocodone Polistirex and Chlorpheniramine Polistirex? Hydrocodone Polistirex and Chlorpheniramine Polistirex can cause serious side effects, including:
-
See “What is the most important information I should know about Hydrocodone Polistirex and Chlorpheniramine Polistirex?”
-
Bowel problems including severe constipation or stomach pain. See “Who should not take Hydrocodone Polistirex and Chlorpheniramine Polistirex?”
-
Increased pressure in your head (intracranial). Avoid the use of Hydrocodone Polistirex and Chlorpheniramine Polistirex if you have a head injury or have been told that you have changes in the tissue of your brain (brain lesions) or increased pressure in your head.
-
Increased risk of seizures in people with seizure disorders. If you have a seizure disorder, Hydrocodone Polistirex and Chlorpheniramine Polistirex may increase how often you have a seizure.
-
Low blood pressure. A sudden drop in blood pressure can happen in some people during treatment with Hydrocodone Polistirex and Chlorpheniramine Polistirex and this may cause you to feel dizzy, faint, lightheaded, or weak, especially when you stand up (orthostatic hypotension). Your risk of having this problem may be increased if you take Hydrocodone Polistirex and Chlorpheniramine Polistirex with certain other medicines that lower blood pressure. If you have any of these symptoms while taking Hydrocodone Polistirex and Chlorpheniramine Polistirex, sit or lie down. Do not change your body position too fast. Get up slowly from sitting or lying down.
Adrenal gland problems. Hydrocodone Polistirex and Chlorpheniramine Polistirex can cause serious and life-threatening adrenal gland problems. Your healthcare provider may do blood tests to check for adrenal gland problems. Call your healthcare provider right away if you have any of these symptoms:
The most common side effects of Hydrocodone Polistirex and Chlorpheniramine Polistirex include:
These are not all the possible side effects of Hydrocodone Polistirex and Chlorpheniramine Polistirex.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
|
|
How should I store Hydrocodone Polistirex and Chlorpheniramine Polistirex?
-
Store Hydrocodone Polistirex and Chlorpheniramine Polistirex at room temperature between 68°F to 77°F (20°C to 25°C).
-
Store Hydrocodone Polistirex and Chlorpheniramine Polistirex in a tightly closed container, in a dry, cool place away from heat or direct sunlight.
-
Keep Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Suspension and all medicines out of the reach of children.
|
|
How should I dispose of Hydrocodone Polistirex and Chlorpheniramine Polistirex?
Remove unused Hydrocodone Polistirex and Chlorpheniramine Polistirex from the container and mix it with an undesirable, non-toxic substance such as cat litter or used coffee grounds to make it less appealing to children and pets. Place the mixture in a container such as a sealed plastic bag and throw it away in the household trash. You can also follow your state or local guidelines on how to safely throw away Hydrocodone Polistirex and Chlorpheniramine Polistirex.
|
|
General information about the safe and effective use of Hydrocodone Polistirex and Chlorpheniramine Polistirex.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Hydrocodone Polistirex and Chlorpheniramine Polistirex for a condition for which it was not prescribed. Do not give Hydrocodone Polistirex and Chlorpheniramine Polistirex to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about Hydrocodone Polistirex and Chlorpheniramine Polistirex that is written for health professionals.
|
|
What are the ingredients in Hydrocodone Polistirex and Chlorpheniramine Polistirex?
Active Ingredients: hydrocodone polistirex and chlorpheniramine polistirex
Inactive Ingredients: ascorbic acid, D&C Yellow No. 10, flavors, high fructose corn syrup, modified food starch, methylparaben, polysorbate 80, polyvinyl acetate, propylene glycol, propylparaben, purified water, sodium ascorbate, sodium metabisulfite, sodium polystyrene sulfonate, sucrose, triacetin, xanthan gum
Manufactured by:
Tris Pharma, Inc.
Monmouth Junction, NJ 08852
LB8535 Rev. 03 05/2021
|
