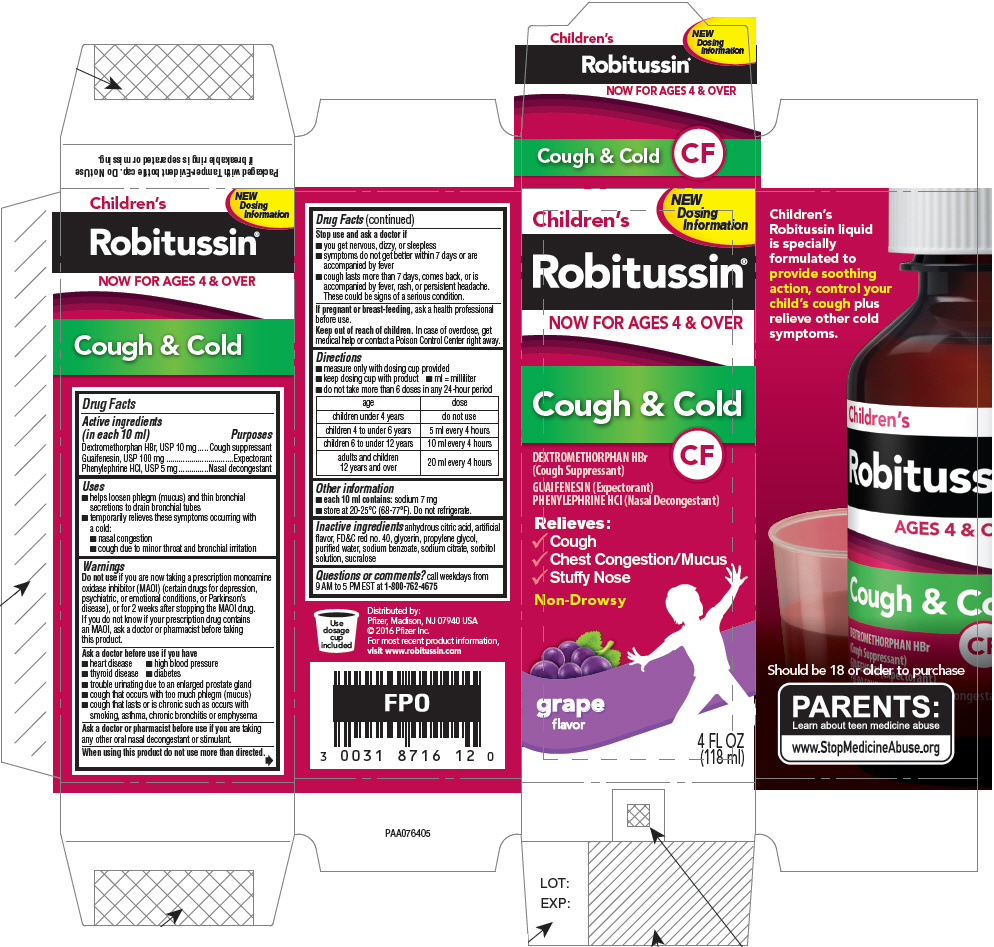
CHILDRENS ROBITUSSIN COUGH AND COLD CF- dextromethorphan hydrobromide, guaifenesin, and phenylephrine hydrochloride liquid
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Active ingredients (in each 10 ml)
Dextromethorphan HBr, USP 10 mg
Guaifenesin, USP 100 mg
Phenylephrine HCl, USP 5 mg
Uses
- 1.
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
- 2.
- temporarily relieves these symptoms occurring with a cold:
- 1.
- nasal congestion
- 2.
- cough due to minor throat and bronchial irritation
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- 1.
- heart disease
- 2.
- high blood pressure
- 3.
- thyroid disease
- 4.
- diabetes
- 5.
- trouble urinating due to an enlarged prostate gland
- 6.
- cough that occurs with too much phlegm (mucus)
- 7.
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Ask a doctor or pharmacist before use if you are taking any other oral nasal decongestant or stimulant.
Directions
- 1.
- measure only with dosing cup provided
- 2.
- keep dosing cup with product
- 3.
- ml = milliliter
- 4.
- do not take more than 6 doses in any 24-hour period
| age | dose |
|---|---|
|
children under 4 years |
do not use |
|
children 4 to under 6 years |
5 ml every 4 hours |
|
children 6 to under 12 years |
10 ml every 4 hours |
|
adults and children 12 years and over |
20 ml every 4 hours |
Other information
- 1.
- each 10 ml contains: sodium 7 mg
- 2.
- store at 20-25°C (68-77°F). Do not refrigerate.
Inactive ingredients
anhydrous citric acid, artificial flavor, FD&C red no. 40, glycerin, propylene glycol, purified water, sodium benzoate, sodium citrate, sorbitol solution, sucralose
Questions or comments?
call weekdays from 9 AM to 5 PM EST at 1-800-762-4675
Distributed by:
Pfizer, Madison, NJ 07940 USA
For most recent product information, visit
www.robitussin.com
PRINCIPAL DISPLAY PANEL
NEW
Dosing
Information
Children's
Robitussin®
NOW FOR AGES 4 & OVER
Cough & Cold
CF
DEXTROMETHORPHAN HBr
(Cough Suppressant)
GUAIFENESIN (Expectorant)
PHENYLEPHRINE HCl (Nasal Decongestant)
Relieves:
- •
- Cough
- •
- Chest Congestion/Mucus
- •
- Stuffy Nose
Non-Drowsy
grape
flavor
4 FL OZ
(118 ml)
| CHILDRENS ROBITUSSIN COUGH AND COLD CF
dextromethorphan hydrobromide, guaifenesin, and phenylephrine hydrochloride liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |