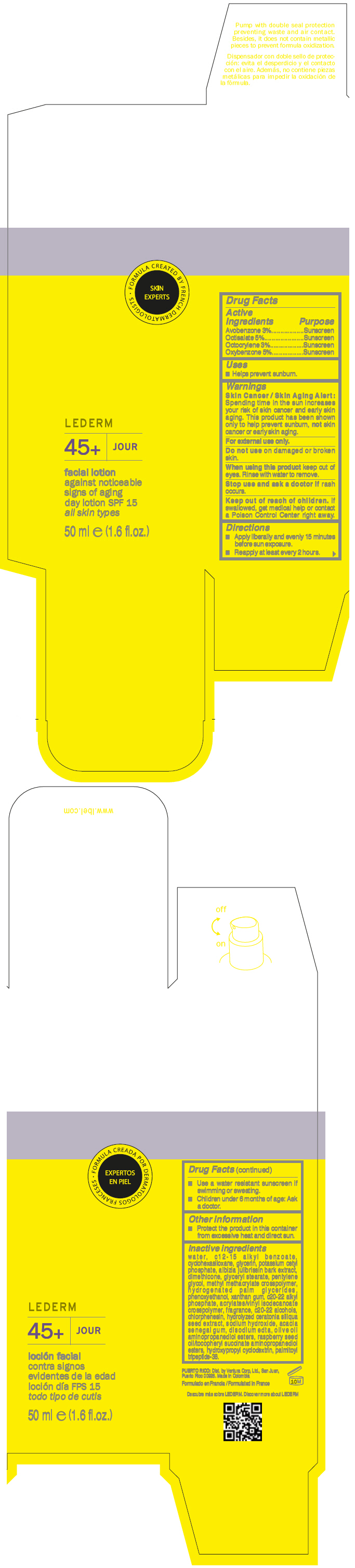
LBEL LEDERM 45 PLUS JOUR FACIAL AGAINST NOTICEABLE SIGNS OF AGING- avobenzone, octisalate, octocrylene, and oxybenzone lotion
Bel Star S.A. (Colombia)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active Ingredients | Purpose |
| Avobenzone 3% | Sunscreen |
| Octisalate 5% | Sunscreen |
| Octocrylene 3% | Sunscreen |
| Oxybenzone 5% | Sunscreen |
Warnings
Skin Cancer / Skin Aging Alert
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn,
not skin cancer or early skin aging.
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: Ask a doctor.
Other information
- Protect the product in this container from excessive heat and direct sun.
Inactive ingredients
water, c12-15 alkyl benzoate, cyclohexasiloxane, glycerin, potassium cetyl phosphate, albizia julibrissin bark extract, dimethicone, glyceryl stearate, pentylene glycol, methyl methacrylate crosspolymer, hydrogenated palm glycerides, phenoxyethanol, xanthan gum, c20-22 alkyl phosphate, acrylates/vinyl isodecanoate crosspolymer, fragrance, c20-22 alcohols, chlorphenesin, hydrolyzed ceratonia siliqua seed extract, sodium hydroxide, acacia senegal gum, disodium edta, olive oil aminopropanediol esters, raspberry seed oil/tocopheryl succinate aminopropanediol esters, hydroxypropyl cyclodextrin, palmitoyl tripeptide-38.
Dist. by Ventura Corp, Ltd., San Juan,
Puerto Rico 00926.
PRINCIPAL DISPLAY PANEL - 50 ml Bottle Box
• FORMULA CREATED BY FRENCH DERMATOLOGISTS
SKIN
EXPERTS
LEDERM
45+ JOUR
facial lotion
against noticeable
signs of aging
day lotion SPF 15
all skin types
50 ml e (1.6 fl.oz.)
Bel Star S.A. (Colombia)