Label: HYFTOR- sirolimus gel
- NDC Code(s): 73683-101-10
- Packager: Nobelpharma America, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HYFTOR™ safely and effectively. See full prescribing information for HYFTOR.
HYFTOR™ (sirolimus topical gel)
Initial U.S. Approval: 1999INDICATIONS AND USAGE
HYFTOR is an mTOR inhibitor immunosuppressant indicated for the treatment of facial angiofibroma associated with tuberous sclerosis in adults and pediatric patients 6 years of age and older. ( 1)
DOSAGE AND ADMINISTRATION
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to HYFTOR initiation. ( 2)
- Apply to the skin of the face affected with angiofibroma twice daily. ( 2)
- The maximum daily dosage is:
- Do not use with occlusive dressings. ( 2)
- For topical use only. Not for oral, ophthalmic, or intravaginal use. ( 2)
DOSAGE FORMS AND STRENGTHS
Topical gel, 0.2%: 2 mg of sirolimus per gram. ( 3)
CONTRAINDICATIONS
History of hypersensitivity to sirolimus or any other component of HYFTOR. ( 4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Oral sirolimus has been associated with hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, angioedema, exfoliative dermatitis, and hypersensitivity vasculitis. Discontinue HYFTOR immediately if symptoms of hypersensitivity occur. ( 5.1)
- Serious Infection: Serious infections, including opportunistic infections and latent viral infections, such as progressive multifocal leukoencephalopathy, have been reported with oral sirolimus. Discontinue HYFTOR immediately if symptoms of infection occur. ( 5.2)
- Malignancy: Oral sirolimus has been associated with malignancy, including lymphoma and skin cancer. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using HYFTOR. ( 5.3)
- Hyperlipidemia: Oral sirolimus has been associated with increased serum cholesterol and triglycerides requiring treatment. Monitor for hyperlipidemia during treatment. ( 5.4)
- Interstitial Lung Disease (ILD)/Non-infectious Pneumonitis: Oral sirolimus has been associated with ILD, sometimes fatal. Discontinue HYFTOR if ILD symptoms occur. ( 5.5)
- Immunizations: During treatment with HYFTOR, vaccinations may be less effective. Avoid use of live vaccines during treatment with HYFTOR. ( 5.6)
- Embryo-Fetal Toxicity: Based on animal studies, HYFTOR can cause fetal harm. Use of effective contraception is recommended for females of reproductive potential prior to and throughout treatment, and for 12 weeks after final dose of HYFTOR. ( 5.7, 8.1, 8.3)
- Male Infertility: Oral sirolimus has been associated with azoospermia and oligospermia. Advise males that HYFTOR may impair fertility. ( 5.8, 8.3, 13.1)
ADVERSE REACTIONS
Most common adverse reactions (≥1%) are dry skin, application site irritation, pruritus, acne, acneiform dermatitis, ocular hyperemia, skin hemorrhage, and skin irritation. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Nobelpharma America, LLC at 1 (877) 375-0825 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CYP3A4 Inhibitors: During concomitant use of HYFTOR with CYP3A4 inhibitors, monitor for adverse reactions of HYFTOR. ( 7.1)
- Substrates and Inhibitors of CYP3A: During concomitant use of HYFTOR with drugs that are both substrates and inhibitors of CYP3A, monitor for adverse reactions of the CYP3A substrate and inhibitor. ( 7.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Serious Infection
5.3 Malignancy
5.4 Hyperlipidemia
5.5 Interstitial Lung Disease/Non-Infectious Pneumonitis
5.6 Immunizations
5.7 Embryo-Fetal Toxicity
5.8 Male Infertility
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on HYFTOR
7.2 Effects of HYFTOR on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to HYFTOR initiation [see Warning and Precautions (5.6)] .
- Apply HYFTOR to the skin of the face affected with angiofibroma twice daily in the morning and at bedtime.
- The maximum recommended daily dosage is:
- 600 mg (2 cm) for pediatric patients 6 to 11 years of age
- 800 mg (2.5 cm) for adults and pediatric patients 12 years of age and older
- If symptoms do not improve within 12 weeks of treatment, reevaluate the need for continuing HYFTOR.
- Do not use HYFTOR with occlusive dressings.
- For topical use only. Not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
HYFTOR is contraindicated in patients with a history of hypersensitivity to sirolimus or any other component of HYFTOR. Reactions to sirolimus have included anaphylactic/anaphylactoid reactions, angioedema, exfoliative dermatitis, and hypersensitivity vasculitis [see Warning and Precautions (5.1)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, angioedema, exfoliative dermatitis, and hypersensitivity vasculitis have been associated with the oral administration of sirolimus. The concomitant use of HYFTOR with other drugs known to cause angioedema, such as angiotensin-converting enzyme (ACE) inhibitors, may increase the risk of developing angioedema. Elevated sirolimus levels (with or without concomitant ACE inhibitors) may also potentiate angioedema. Discontinue HYFTOR immediately if symptoms of hypersensitivity occur.
5.2 Serious Infection
Serious infections, including opportunistic infections, have been reported after oral administration of sirolimus. Cases of progressive multifocal leukoencephalopathy (PML), sometimes fatal have been reported in patients treated with oral sirolimus. Discontinue HYFTOR immediately if symptoms of infection occur.
5.3 Malignancy
Lymphoma and other malignancies, particularly of the skin, have been observed after oral administration of sirolimus. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using HYFTOR. If patients need to be outdoors while using HYFTOR, they should wear protective clothing and discuss other sun protection measures with their physician.
5.4 Hyperlipidemia
Increased serum cholesterol and triglycerides requiring treatment have been observed with oral administration of sirolimus. Monitor for hyperlipidemia during treatment with HYFTOR.
5.5 Interstitial Lung Disease/Non-Infectious Pneumonitis
Cases of interstitial lung disease [ILD] (including pneumonitis, bronchiolitis obliterans organizing pneumonia [BOOP], and pulmonary fibrosis), some fatal, with no identified infectious etiology have occurred in patients receiving oral sirolimus. In some cases, the ILD has resolved upon discontinuation or dosage reduction of oral sirolimus. Discontinue HYFTOR immediately if symptoms of ILD occur.
5.6 Immunizations
During treatment with HYFTOR, vaccinations may be less effective. Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating treatment with HYFTOR. The use of live vaccines should be avoided during treatment with HYFTOR.
5.7 Embryo-Fetal Toxicity
Based on animal studies and the mechanism of action, oral sirolimus can cause fetal harm when administered to a pregnant woman. In animal studies, oral sirolimus caused embryo-fetal toxicity when administered during the period of organogenesis at maternal exposures that were equal to or less than human exposures at the recommended lowest starting dose. HYFTOR is systemically absorbed after topical administration and may result in fetal exposure. Advise pregnant women of the potential risk to a fetus. Advise female patients of reproductive potential to avoid becoming pregnant. They should use effective contraception prior to, throughout treatment and for 12 weeks after the final dose of HYFTOR [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1, 12.3)].
5.8 Male Infertility
Azoospermia or oligospermia has been observed after oral administration of sirolimus [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)]. Sirolimus is an anti-proliferative drug and affects rapidly dividing cells like the germ cells. Advise males that HYFTOR may impair fertility.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a randomized, double-blind, vehicle-controlled trial, subjects aged 6 years and older with facial angiofibroma associated with tuberous sclerosis applied HYFTOR twice daily for 12 weeks. A total of 30 subjects were treated with HYFTOR and 32 with the vehicle. The majority of the subjects were female (54.8%). A total of 40.3% were less than 18 years of age.
The most common adverse reactions reported by ≥1% of subjects treated with HYFTOR and more frequently than in subjects treated with vehicle are presented in Table 1. Adverse reactions occurred with similar frequency in pediatric subjects 6 years of age and older.
Table 1: Adverse Reactions in ≥1% of Subjects Aged 6 Years and Older with Facial Angiofibroma Associated with Tuberous Sclerosis Through Week 12 Preferred Term HYFTOR
N = 30Vehicle
N = 32- *
- Dry skin includes dry skin and asteatosis
Dry skin * 12 (40%) 4 (13%) Application site irritation 11 (37%) 9 (28%) Pruritus 5 (17%) 4 (13%) Acne 2 (7%) 0 (0%) Acneiform dermatitis 1 (3%) 0 (0%) Ocular hyperemia 1 (3%) 0 (0%) Skin hemorrhage 1 (3%) 0 (0%) Skin irritation 1 (3%) 0 (0%) In a 104-week, open-label safety trial, the most common adverse reactions associated with HYFTOR application were application site irritation (31%), dry skin (28%), acne (20%), pruritus (9%), eye irritation (9%), erythema (7%), acneiform dermatitis (6%), contact dermatitis (5%), solar dermatitis (1%), and photosensitivity reaction (1%). Adverse reactions occurred with similar frequency in adult and pediatric subjects 6 years of age and older.
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on HYFTOR
Table 2 presents clinically significant drug interactions involving drugs that affect HYFTOR.
Table 2: Effects of CYP3A4 Inhibitors on HYFTOR Clinical Impact Concomitant use of HYFTOR with inhibitors of CYP3A4 has the potential to increase the systemic exposure of sirolimus and increase the risk of HYFTOR adverse reactions. Intervention Monitor for adverse reactions of HYFTOR. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal studies and mechanism of action, oral sirolimus can cause fetal harm when administered to a pregnant woman. HYFTOR is systemically absorbed after topical administration and may result in fetal exposure. The available data from case reports on HYFTOR use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with HYFTOR. In an animal reproduction study, oral administration of 0.5 mg/kg/day sirolimus caused embryo-fetal lethality in pregnant rats when administered during the period of organogenesis (see Data). The available data do not allow the calculation of relevant comparisons between the systemic exposure of sirolimus observed in animal studies to the systemic exposure that would be expected in humans after topical use of HYFTOR.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In embryo-fetal development studies in rats, oral administration of sirolimus to pregnant rats during the period of organogenesis (gestation days 6 -15) induced embryo-fetal lethality at 0.5 mg/kg/day, reduced fetal body weight at 1.0 mg/kg/day and caused maternal toxicity at 2.0 mg/kg/day. No treatment related embryo-fetal developmental effects were observed at 0.1 mg/kg/day.
In embryo-fetal development studies in rabbits, oral administration of sirolimus to pregnant rabbits during the period of organogenesis (gestation days 6 -18) induced maternal toxicity (decreased body weight) at 0.05 mg/kg/day, which was associated with embryo-fetal loss or early resorption. No treatment related developmental effects were observed at 0.025 mg/kg/day.
In a pre- and postnatal development toxicity study, oral administration of sirolimus to pregnant rats during gestation and lactation (gestation day 6 through lactation day 20) increased the incidence of dead pups, resulting in reduced live litter size at 0.5 mg/kg/day. No treatment related developmental effects were observed at 0.1 mg/kg/day.
Sirolimus did not cause maternal toxicity or affect developmental parameters in the surviving offspring (morphological development, motor activity, learning, or fertility assessment) at 0.5 mg/kg/day, the highest dose tested.
8.2 Lactation
Risk Summary
There are no available data on the presence of sirolimus in human milk, the effects on the breastfed infant, or the effects on milk production. After oral administration, sirolimus was present in the milk of lactating rats. Because of the potential for serious adverse reactions in the breastfed infant, breastfeeding is not recommended during treatment with HYFTOR.
8.3 Females and Males of Reproductive Potential
Contraception
Based on animal studies with oral sirolimus, HYFTOR may cause fetal harm when administered to pregnant women. Females of reproductive potential are recommended to avoid becoming pregnant and to use an effective contraceptive method. Effective contraception should be initiated before HYFTOR therapy and used throughout treatment and for 12 weeks after the final dose of HYFTOR [see Warnings and Precautions (5.7), Use in Specific Populations (8.1)] .
Infertility
Based on clinical findings and findings in animal studies, male and female fertility may be compromised by the treatment with sirolimus [see Warnings and Precautions (5.8), Nonclinical Toxicology (13.1)]. Ovarian cysts and menstrual disorders (including amenorrhea and menorrhagia) have been reported in females with the use of oral sirolimus. Azoospermia has been reported in males with the use of oral sirolimus and have been reversible upon discontinuation of sirolimus in most cases.
8.4 Pediatric Use
The safety and effectiveness of HYFTOR have been established in pediatric patients aged 6 years and older for the topical treatment of facial angiofibroma associated with tuberous sclerosis. Use of HYFTOR in this age group is supported by data from a randomized, vehicle-controlled, double-blind 12-week trial along with additional efficacy and long-term safety data from a 104-week open label safety trial. A total of 13 pediatric subjects aged 6 years to 17 years received HYFTOR in the Phase 3 clinical trial along with 48 pediatric subjects aged 3 years to 17 years in the 104-week open label safety trial. Adverse reactions occurred with similar frequency in adult and pediatric subjects [see Adverse Reaction (6.1), Clinical Studies (14)].
The safety and effectiveness of HYFTOR for this indication have not been established in pediatric patients less than 6 years of age.
8.5 Geriatric Use
Clinical studies of HYFTOR did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
11 DESCRIPTION
HYFTOR™ (sirolimus topical gel) 0.2% is an mTOR inhibitor immunosuppressant for topical use. Each gram contains 2 mg of sirolimus, which is solubilized in a gel consisting of alcohol 51%, Carbomer 940, purified water, and trolamine.
Chemically, sirolimus is designated as (3 S,6 R,7 E,9 R,10 R,12 R,14 S,15 E,17 E,19 E,21 S,23 S,26 R,27 R,34a S)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-9,27-dihydroxy-3-[(1 R)-2-[(1 S,3 R,4 R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3 H-pyrido[2,1- c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4 H,6 H,31 H)-pentone. It has the following structural formula:
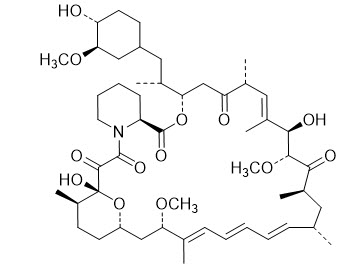
Sirolimus is a white to off-white powder and is insoluble in water, but freely soluble in chloroform, acetone and acetonitrile. Sirolimus has a molecular formula of C 51H 79NO 13 and a molecular weight of 914.19.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of sirolimus in the treatment of angiofibroma associated with tuberous sclerosis is unknown. Tuberous sclerosis is associated with genetic defects in TSC1 and TSC2 which leads to the constitutive activation of mammalian target of rapamycin (mTOR). Sirolimus inhibits mTOR activation.
12.3 Pharmacokinetics
Absorption
Following 12 weeks of treatment with HYFTOR in adult and pediatric subjects aged 6 years and older, sirolimus blood concentrations ranged from undetectable to 0.50 ng/mL after multiple doses of HYFTOR in the Phase 3 trial. Periodic blood samples were obtained in the 52-week trial and the maximum sirolimus concentration measured at any time in adult subjects was 3.27 ng/mL and the maximum sirolimus concentration measured at any time in pediatric subjects was 1.80 ng/mL.
Distribution
There was no evidence based on blood concentrations that sirolimus accumulates systemically upon topical application in patients with tuberous sclerosis for periods of up to 1 year.
Elimination
Metabolism
Studies evaluating the metabolism of HYFTOR have not been conducted.
Sirolimus is a substrate for both CYP3A4 and P-gp. Sirolimus is extensively metabolized after oral administration in the intestinal wall and liver and undergoes counter-transport from enterocytes of the small intestine into the gut lumen. Inhibitors of CYP3A4 and P-gp increase sirolimus concentrations. Inducers of CYP3A4 and P-gp decrease sirolimus concentrations. Sirolimus is extensively metabolized by O-demethylation and/or hydroxylation. Seven (7) major metabolites, including hydroxy, demethyl, and hydroxydemethyl, are identifiable in whole blood. Some of these metabolites are also detectable in plasma, fecal, and urine samples. Sirolimus is the major component in human whole blood and contributes to more than 90% of the immunosuppressive activity.
Excretion
Studies evaluating the excretion of HYFTOR have not been conducted.
After a single dose of [ 14C] sirolimus oral solution in healthy volunteers, the majority (91%) of radioactivity was recovered from the feces, and only a minor amount (2.2%) was excreted in urine. The mean ± SD terminal elimination half-life (t½) of sirolimus after multiple dosing in stable renal transplant patients was estimated to be about 62 ± 16 hours.
Drug Interaction Studies
Drug interaction studies with HYFTOR have not been conducted. Sirolimus is known to be a substrate for both cytochrome P-450 3A4 (CYP3A4) and p-glycoprotein (P-gp). [see Drug Interactions (7.1, 7.2)] .
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Oral carcinogenicity studies were conducted in mice and rats. In an oral carcinogenicity study conducted in female mice, sirolimus administered once daily for 86 weeks was associated with a statistically significant increase in malignant lymphoma at all dose levels compared with control animals. In a second oral mouse carcinogenicity study, sirolimus-related hepatocellular adenoma and carcinoma were induced in male mice. In an oral carcinogenicity study conducted in rats with sirolimus there were no significant findings.
Sirolimus was not genotoxic in the in vitro bacterial reverse mutation assay, the Chinese hamster ovary cell chromosomal aberration assay, the mouse lymphoma cell forward mutation assay, or the in vivo mouse micronucleus assay.
Fertility was decreased in both male and female rats following oral administration of sirolimus 2.0 and 0.5 mg/kg, respectively. In male rats, atrophy of testes, epididymides, prostate, seminiferous tubules and/or reduced sperm counts were observed. In female rats, decreased ovarian and uterine weights and decreased implantation were observed. Testicular tubular degeneration was also seen in a 4-week intravenous study of sirolimus in monkeys at 0.1 mg/kg.
-
14 CLINICAL STUDIES
A single, randomized, double-blind, vehicle-controlled, multi-center, Phase 3 trial was conducted in Japan to evaluate HYFTOR for the treatment of adults and pediatric patients 6 years of age and older with facial angiofibroma associated with tuberous sclerosis (NCT02635789). A total of 62 Japanese subjects with 3 or more angiofibromas (≥2 mm in diameter with redness in each) on the face were enrolled in this trial. Overall, 28 subjects (45%) were male and 34 (55%) were female. A total of 25 subjects (40%) were between 6 and <18 years of age. In this trial, subjects applied either HYFTOR or vehicle twice daily to the skin of their face affected with angiofibroma for 12 weeks.
The efficacy was assessed by the investigator (live assessment) based on the composite improvement from baseline in size and redness of facial angiofibroma, using subjects' baseline photographs as reference. The proportion of subjects assessed as 'Improved' or 'Markedly Improved' at Week 12 is presented in Table 3. An assessment of 'Improved' was defined as at least a 50% reduction in the size and a 2-level reduction in redness and an assessment of 'Markedly Improved' was defined as at least a 75% reduction in the size and a 3-level reduction in redness.
Table 3: Improvement in Facial Angiofibroma Associated with Tuberous Sclerosis in Patients Aged 6 Years and Older at Week 12 Proportion of Subjects Assessed by the Investigator as: HYFTOR
N=30Vehicle
N=32'Improved' or 'Markedly Improved' 23% 6% 'Improved' 13% 3% 'Markedly Improved' 10% 3% - 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity
Inform patients that oral sirolimus has been associated with hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, angioedema, exfoliative dermatitis, and hypersensitivity vasculitis. Advise patients to discontinue HYFTOR immediately and seek medical attention if symptoms occur [see Warnings and Precautions (5.1)] .
Serious Infections
Inform patients that oral sirolimus has been associated with increased susceptibility to infections, including opportunistic infections and latent viral infections, such as progressive multifocal leukoencephalopathy (PML). Advise patients to discontinue HYFTOR immediately and seek medical attention if symptoms occur [see Warnings and Precautions (5.2)] .
Malignancy
Inform patients that oral sirolimus has been associated with malignancy, including lymphoma and skin cancer. Advise patients to minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment), and to use protective measures if exposure cannot be avoided, while using HYFTOR [see Warnings and Precautions (5.3)] .
Hyperlipidemia
Inform patients that oral sirolimus has been associated with increased serum cholesterol and triglycerides requiring treatment and that periodic laboratory monitoring may be needed [see Warnings and Precautions (5.4)] .
Interstitial Lung Disease
Inform patients that oral sirolimus has been associated with interstitial lung disease [ILD] sometimes fatal, with no identified infectious etiology. Advise patients to discontinue HYFTOR immediately and seek medical attention if symptoms (e.g. shortness of breath) occur [see Warnings and Precautions (5.5)] .
Immunization
Inform patients that during treatment with HYFTOR, vaccinations may be less effective. Instruct patients that vaccination with live vaccines should be avoided during treatment with HYFTOR and to inform the healthcare practitioner that they are using HYFTOR prior to a potential vaccination [see Warnings and Precautions (5.6)] .
Pregnancy
HYFTOR may cause fetal harm if used during pregnancy. Advise female patients of reproductive potential to avoid becoming pregnant and to use effective contraception prior to, throughout treatment, and for 12 weeks after the final dose of HYFTOR. Advise pregnant women of the potential risk to a fetus [see Warnings and Precautions (5.7), Use in Specific Populations (8.1, 8.3)].
Lactation
Advise lactating women that breastfeeding is not recommended during treatment with HYFTOR [see Use in Specific Populations (8.2)].
Infertility
Inform male and female patients that HYFTOR may impair fertility [see Warnings and Precautions (5.8), Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Administration Information
Inform patients that the skin being treated with HYFTOR should not be covered with bandages, dressings or wraps [see Dosage and Administration (2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 2/2022 PATIENT INFORMATION
HYFTOR™ (hyfe tore)
(sirolimus topical gel)Important: HYFTOR is for use on the skin only (topical use). Do not use HYFTOR in your mouth, eyes, or vagina. What is HYFTOR?
HYFTOR is a prescription medicine that is used on the skin (topical) to treat adults and children 6 years of age and older with a type of noncancerous tumor called angiofibroma on your face caused by the genetic condition tuberous sclerosis.
It is not known if HYFTOR is safe and effective in children under 6 years of age.Do not use HYFTOR if you are allergic to sirolimus or any of the other ingredients in HYFTOR. See the end of this leaflet for a complete list of ingredients in HYFTOR. Before using HYFTOR, tell your healthcare provider about all of your medical conditions, including if you: - have a skin infection at the treatment site
- have high cholesterol or high triglycerides (fat or lipids) in your blood
- are scheduled to receive an immunization (vaccine). You should avoid receiving live vaccines during treatment with HYFTOR. Vaccines may be less effective during treatment with HYFTOR.
- are pregnant or plan to become pregnant. HYFTOR may harm your unborn baby. You should not become pregnant during treatment with HYFTOR.
- Females who are able to become pregnant should use effective birth control (contraception) before starting treatment with HYFTOR, during treatment, and for 12 weeks after your final dose of HYFTOR. Talk to your healthcare provider about types of birth control that you can use during this time.
- are breastfeeding or plan to breastfeed. It is not known if HYFTOR passes into your breast milk. You should not breastfeed during treatment with HYFTOR.
How should I use HYFTOR? - Use HYFTOR exactly as your healthcare provider tells you to use it.
- Before you use HYFTOR, your healthcare provider or pharmacist should show you how to correctly measure your dose.
- Wash your hands before and after applying HYFTOR.
- Apply HYFTOR to the skin of the face affected with angiofibroma 2 times a day, in the morning and at bedtime.
- Do not cover, wrap, apply dressings, or bandage the skin area treated with HYFTOR.
- Tell your healthcare provider if the treated skin area does not improve within 12 weeks of treatment.
What should I avoid while using HYFTOR?
Limit your exposure to sunlight and ultraviolet light, such as tanning beds and ultraviolet light therapy, during treatment with HYFTOR. Wear clothing that covers your skin if you need to go outside. Talk with your healthcare provider about other ways you can protect your skin from the sun.What are the possible side effects of HYFTOR?
HYFTOR may cause serious side effects, including:- Allergic reactions. Serious allergic reactions have happened in people who have taken sirolimus by mouth. Stop using HYFTOR and get medical help right away if you get any of these symptoms of a serious allergic reaction:
- swelling of your face, eyes, or mouth
- trouble breathing or wheezing
- throat tightness
- chest pain or tightness
- feeling dizzy or faint
- rash or peeling of your skin
- Infections. Serious infections, including infections that can happen when your immune system is weak, have happened in people who have taken sirolimus by mouth. Some people have developed a rare, serious brain infection called progressive multifocal leukoencephalopathy (PML) which can sometimes cause death. Stop using HYFTOR and call your healthcare provider right away if you get symptoms of an infection including fever or chills.
- Risk of cancer. Lymphoma and other cancers, especially skin cancer, have happened in people who have taken sirolimus by mouth. Talk with your healthcare provider about your risk for cancer if you use HYFTOR.
- Increased levels of cholesterol and triglycerides (fat or lipids) in the blood have happened in people who have taken sirolimus by mouth . Your healthcare provider may do blood tests to check you for high lipid levels during treatment with HYFTOR and treat you, if needed.
- Lung or breathing problems. Lung or breathing problems, including problems that have sometimes caused death, have happened in people who have taken sirolimus by mouth. Stop using HYFTOR and get medical help right away if you get symptoms such as shortness of breath, new or worsening cough, or chest pain.
HYFTOR may cause fertility problems in males and females, which may affect your ability to have children. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of HYFTOR.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store HYFTOR ? - Store HYFTOR in the refrigerator between at 36°F to 46°F (2°C to 8°C).
- Keep HYFTOR out of light.
General information about the safe and effective use of HYFTOR.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use HYFTOR for a condition for which it was not prescribed. Do not give HYFTOR to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about HYFTOR that is written for health professionals.What are the ingredients in HYFTOR?
Active ingredient: sirolimus
Inactive ingredients: alcohol 51%, Carbomer 940, purified water, and trolamine.
Distributed by: Nobelpharma America, LLC
4520 East-West Highway, Suite 400, Bethesda, MD 20814
For more information, go to www.Hyftor.com or call 887-375-0825. - PRINCIPAL DISPLAY PANEL - 10 g Tube Carton
-
INGREDIENTS AND APPEARANCE
HYFTOR
sirolimus gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73683-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SIROLIMUS (UNII: W36ZG6FT64) (SIROLIMUS - UNII:W36ZG6FT64) SIROLIMUS 2 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) ALCOHOL (UNII: 3K9958V90M) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73683-101-10 1 in 1 CARTON 03/23/2022 1 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213478 03/23/2022 Labeler - Nobelpharma America, LLC (117340493) Establishment Name Address ID/FEI Business Operations Sharp Clinical, Inc 079209266 analysis(73683-101) , label(73683-101) , pack(73683-101) Establishment Name Address ID/FEI Business Operations Toyo Pharmaceutical Co., Ltd. 717792357 manufacture(73683-101) , analysis(73683-101)


