Label: PROHEART 6- moxidectin kit
- NDC Code(s): 54771-3670-1, 54771-3671-1, 54771-3672-1
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated March 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- CAUTION
-
DESCRIPTION
ProHeart 6 (moxidectin) for extended-release injectable suspension consists of two separate vials: One vial contains 10% moxidectin sterile microspheres and the second vial contains a specifically formulated sterile vehicle for constitution with the microspheres. No other diluent should be used. A clear or translucent appearance of the vehicle is normal. Each mL of constituted drug product contains 3.4 mg moxidectin, 3.1% glyceryl tristearate, 2.4% hydroxypropyl methylcellulose, 0.87% sodium chloride, 0.17% methylparaben, 0.02% propylparaben and 0.001% butylated hydroxytoluene. Hydrochloric acid is used to adjust pH. The constituted product may appear as a hazy to milky suspension.
- INDICATIONS
-
DOSAGE AND ADMINISTRATION
Always provide Client Information Sheet and review with owners before administering ProHeart 6. The owner should be advised to observe their dog for adverse drug events including those described on the sheet.
Frequency of Treatment
ProHeart 6 prevents the development of heartworm disease caused by D. immitis for six months. It should be administered within one month of the dog’s first exposure to mosquitoes. Follow-up treatments may be given every six months if the dog has continued exposure to mosquitoes and if the dog continues to be healthy without weight loss. When replacing another heartworm preventive product, ProHeart 6 should be given within one month of the last dose of the former medication.
ProHeart 6 eliminates the larval and adult stages of A. caninum and U. stenocephala present at the time of treatment. However, persistent effectiveness has not been established for this indication. Re-infection with A. caninum and U. stenocephala may occur sooner than 6 months.Dose
The recommended subcutaneous dose is 0.05 mL of the constituted suspension/kg body weight (0.0227 mL/lb.). This amount of suspension will provide 0.17 mg moxidectin/ kg bodyweight (0.0773 mg/lb.).
To ensure accurate dosing, calculate each dose based on the dog’s weight at the time of treatment. Do not overdose growing puppies in anticipation of their expected adult weight. The following dosage chart may be used as a guide.DOSAGE CHART Dog Wt. Dose Volume Dog Wt. Dose Volume lb kg mL/Dog lb kg mL/Dog 11 5 0.25 77 35 1.75 22 10 0.50 88 40 2.00 33 15 0.75 99 45 2.25 44 20 1.00 110 50 2.50 55 25 1.25 121 55 2.75 66 30 1.50 132 60 3.00 Injection Technique
The two-part sustained release product must be mixed at least 30 minutes prior to the intended time of use (see CONSTITUTION PROCEDURES for initial mixing instructions). Once constituted, swirl the bottle gently before every use to uniformly re-suspend the microspheres. Withdraw 0.05 mL of suspension/kg body weight into an appropriately sized syringe fitted with an 18G or 20G hypodermic needle. Dose promptly after drawing into dosing syringe. If administration is delayed, gently roll the dosing syringe prior to injection to maintain a uniform suspension and accurate dosing.
Using aseptic technique, inject the product subcutaneously in the left or right side of the dorsum of the neck cranial to the scapula. No more than 3 mL should be administered in a single site. The location(s) of each injection (left or right side) should be noted so that prior injection sites can be identified and the next injection can be administered on the opposite side. -
RISK MINIMIZATION ACTION PLAN
The ProHeart 6 and ProHeart 12 Risk Minimization Action Plan (RiskMAP) provides educational materials to the veterinarian, veterinary staff, and the dog owner explaining the risks and proper use of ProHeart 6 and ProHeart 12. ProHeart 6 and ProHeart 12 are the same formulation, but ProHeart 6 is three times less concentrated than ProHeart 12. ProHeart 6 and ProHeart 12 are for use in dogs only and are available through a restricted distribution program to veterinarians that have completed the RiskMAP training and certification module.
The ProHeart 6 and ProHeart 12 web-based training and certification module is available at http://www.proheart6.com. This website has important information on the safe and effective use of ProHeart 6 and ProHeart 12 for veterinarians.
Only veterinarians and veterinary technicians/assistants that have completed the training and are certified can administer ProHeart 6 and ProHeart 12. Veterinarians are expected to report all adverse events that occur in animals or humans to the manufacturer. Important safety information is included below: - CONTRAINDICATIONS
-
HUMAN WARNINGS
Not for human use. Keep this and all drugs out of the reach of children.
May be slightly irritating to the eyes. May cause slight irritation to the upper respiratory tract if inhaled. May be harmful if swallowed. If contact with the eyes
occurs, rinse thoroughly with water for 15 minutes and seek medical attention immediately. If accidental ingestion occurs, contact a Poison Control Center
or a physician immediately. The Safety Data Sheet (SDS) contains more detailed occupational safety information. -
WARNINGS
Anaphylactic and anaphylactoid reactions may occur in some dogs following administration of ProHeart 6 alone or with vaccines. In some cases, these reactions have resulted in death following administration of moxidectin microspheres (see POST-APPROVAL EXPERIENCE). Anaphylactic and anaphylactoid reactions should be treated immediately with the same measures used to treat hypersensitivity reactions to vaccines and other injectable products.
Always provide Client Information Sheet and review with owners before administering ProHeart 6. The owner should be advised to observe their dog for adverse drug events including those described on the sheet.
Do not administer ProHeart 6 to dogs who are sick, debilitated, underweight or who have a history of weight loss. -
PRECAUTIONS
Prior to administration of ProHeart 6, the health of the patient should be assessed by a thorough medical history, physical examination and diagnostic testing as indicated (see WARNINGS).
Caution should be used when administering ProHeart 6 in dogs with pre-existing allergic disease, including food allergy, atopy, and flea allergy dermatitis. (see WARNINGS).
Caution should be used when administering ProHeart 6 concurrently with vaccinations. Adverse reactions, including anaphylaxis, have been reported following the concomitant use of moxidectin microspheres and vaccinations (see WARNINGS and POST-APPROVAL EXPERIENCE).
ProHeart 6 should not be used more frequently than every 6 months.
The effectiveness of ProHeart 6 has not been evaluated in dogs less than 6 months of age.
Prior to administration of ProHeart 6, dogs should be tested for existing heartworm infections. Infected dogs should be treated with an adulticide to remove adult heartworms. ProHeart 6 is not effective against adult D. immitis.
Caution should be used when administering ProHeart 6 to heartworm positive dogs (see ADVERSE REACTIONS). -
ADVERSE REACTIONS
In field studies, the following adverse reactions were observed in dogs treated with ProHeart 6: anaphylaxis, vomiting, diarrhea (with and without blood), listlessness, weight loss, seizures, injection site pruritus, and elevated body temperature. Dogs with clinically significant weight loss (>10%) were more likely to experience a severe adverse reaction.
In a laboratory effectiveness study, dogs with 4- and 6-month-old heartworm infections experienced vomiting, lethargy and bloody diarrhea. These signs were more severe in the dogs with 4-month-old heartworm infections, including one dog that was recumbent and required supportive care, than in the dogs with older (6-month-old) infections.
Post-Approval Experience (Rev. 2018)
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse reactions are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data. The following adverse events are listed in decreasing order of frequency by body system.
Immune: anaphylaxis and/or anaphylactoid reactions, urticaria, head/facial edema, pruritus, pale mucous membranes, collapse, cardiovascular shock, erythema, immune-mediated hemolytic anemia, immune-mediated thrombocytopenia (signs reflected in other system categories could be related to allergic reactions, i.e. gastrointestinal, dermatologic, and hematologic)
Gastrointestinal: vomiting (with or without blood), diarrhea with or without blood, hypersalivation
General: depression, lethargy, anorexia, fever, weight loss, weakness
Dermatological: injection site pruritus/swelling, erythema multiforme
Neurological: seizures, ataxia, trembling, hind limb paresis
Hematological: leukocytosis, anemia, thrombocytopenia
Respiratory: dyspnea, tachypnea, coughing
Hepatic: elevated liver enzymes, hypoproteinemia, hyperbilirubinemia, hepatopathy
Urinary: elevated BUN, elevated creatinine, hematuria, polydipsia, polyuria
Cardiopulmonary signs such as coughing and dyspnea may occur in heartworm positive dogs.
In some cases, death has been reported as an outcome of the adverse events listed above.
For a copy of the Safety Data Sheet (SDS) or to report suspected adverse reactions, contact Zoetis at 1-888-963-8471. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae. -
INFORMATION FOR DOG OWNERS
Always provide Client Information Sheet and review with owners before administering ProHeart 6. Owners should be advised of the potential for adverse reactions, including anaphylaxis, and be informed of the clinical signs associated with drug toxicity (see WARNINGS, ADVERSE REACTIONS and POST-APPROVAL EXPERIENCE sections.)
Owners should be advised to contact their veterinarian immediately if signs of toxicity are observed. The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized and veterinary care, if appropriate, is initiated.
-
CLINICAL PHARMACOLOGY
Moxidectin is a semi-synthetic methoxime derivative of nemadectin which is a fermentation product of Streptomyces cyaneogriseus subspecies noncyanogenus. Moxidectin is a pentacyclic 16-membered lactone macrolide.
Moxidectin has activity resulting in paralysis and death of affected parasites. The stage of the canine heartworm affected at the recommended dose rate of 0.5 mg/kg (0.227 mg/lb) is the tissue larval stage.
The larval and adult stages of the canine hookworms, A. caninum and U. stenocephala, are susceptible.
Following injection with ProHeart 6, peak moxidectin blood levels will be observed approximately 7-14 days after treatment. At the end of the 6-month dosing interval, residual drug plasma concentrations are negligible. Accordingly, little or no drug accumulation is expected to occur with repeated administrations. -
ANIMAL SAFETY
General Safety: ProHeart 6 has been administered to a wide variety of healthy dogs six months of age and older, including a wide variety of breeds, pregnant and lactating females, breeding males, and ivermectin-sensitive collies. In clinical studies, two geriatric dogs with a history of weight loss after the initial ProHeart 6 injection died within a month of the second 6 month injection. A third dog who was underweight for its age and breed and who had a history of congenital problems experienced lethargy following the initial injection of ProHeart 6. The dog never recovered and died 3 months later (see WARNINGS).
ProHeart 6 administered at 3 times the recommended dose in dogs with patent heartworm infections and up to 5 times the recommended dose in ivermectin-sensitive collies did not cause any adverse reactions. ProHeart 6 administered at 3 times the recommended dose did not adversely affect the reproductive performance of male or female dogs.
ProHeart 6 administered up to 5 times the recommended dose in 7-8 month old puppies did not cause any systemic adverse effects.
In well controlled clinical field studies, ProHeart 6 was used in conjunction with a variety of veterinary products including anthelmintics, antiparasitics, antibiotics, analgesics, steroids, non-steroidal anti-inflammatory drugs (NSAIDs), anesthetics and flea control products.
Injection Site Reactions: Injection site observations were recorded during effectiveness and safety studies. In clinical studies, ProHeart 6 was administered at six-month intervals to client-owned dogs under field conditions. There were no reports of injection site reactions in these field studies and evaluations of the injection sites revealed no abnormalities.
In a laboratory safety study, ProHeart 6 was administered at 1, 3 and 5 times the recommended dose to 7-8 month old puppies. Injection sites were clipped to facilitate observation. Slight swelling/edema at the injection site was observed in some dogs from all treated groups. These injection site reactions appeared as quickly as 8 hours post injection and lasted up to 3 weeks. A three-year repeated injection study was conducted to evaluate the safety of up to 6 injections of ProHeart 6 administered at the recommended dose (0.17 mg/kg) every 6 months. Mild erythema and localized deep subcuticular thickening were seen in dogs that received four injections in the same area on the neck and in one dog that received two injections in the same area on the neck. Microscopic evaluation on the injection sites from all dogs 6 months after the last injection consistently showed mild granulomatous panniculitis with microvacuolation. The only adverse reaction seen that was not related to the injection site was weight loss in one dog.
Some dogs treated with ProHeart 6 in laboratory effectiveness studies developed transient, localized inflammatory injection site reactions. These injection site reactions were visible grossly for up to 3 weeks after injection. Histologically, well-defined granulomas were observed in some dogs at approximately 5 months after injection.
-
CONSTITUTION PROCEDURES
The two-part ProHeart 6 product must be mixed at least 30 minutes prior to the intended time of use.
Items needed to constitute ProHeart 6: - Microspheres
- Sterile 20 mL syringe for transfer
- Enclosed vent needle (25G)
- Transfer needle (18G or 20G)
- Sterile Vehicle
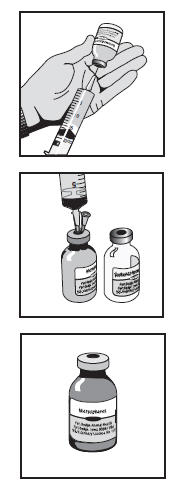
Constitution of the 20 mL vial product (17.7 mL when constituted). 1. Shake the microsphere vial to break up any aggregates prior to constitution. 2. Using an 18G or 20G needle and sterile syringe withdraw 17.0 mL of the unique sterile vehicle from the vial. There is more sterile vehicle supplied than the 17.0 mL required. 3. Insert the enclosed 25G vent needle into the microsphere vial. 4. Slowly transfer the sterile vehicle into the microsphere vial through the stopper using the transfer needle and syringe. 5. Once the sterile vehicle has been added, remove the vent and transfer needles from the microsphere vial. Discard unused sterile vehicle and needles. 6. Shake the microsphere vial vigorously until a thoroughly mixed suspension is produced. The constituted product may appear as a hazy to milky suspension. 7. Record the time and date of mixing on the microsphere vial. 8. Allow suspension to stand for at least 30 minutes to allow large air bubbles to dissipate. 9. Before every use, gently swirl the mixture to achieve uniform suspension. The constituted product may appear as a hazy to milky suspension. The microspheres and vehicle will gradually separate on standing. 10. Use a 1 mL or 3 mL syringe and an 18G or 20G needle for dosing. Dose promptly after drawing into dosing syringe. If administration is delayed, gently roll the dosing syringe prior to injection to maintain a uniform suspension and accurate dosing. 11. Refrigerate the unused product. The constituted product remains stable for 8 weeks in a refrigerator. Avoid direct sunlight. - STORAGE INFORMATION
-
HOW SUPPLIED
ProHeart 6 is available in the following three package sizes.
1. 1-Pack 2. 5-Pack 3. 10-Pack 20 mL vial product: 20 mL vial product: 20 mL vial product: 1 - 10% moxidectin sterile microspheres 5 - 10% moxidectin sterile microspheres 10 - 10% moxidectin sterile microspheres - 598 mg/vial - 598 mg/vial - 598 mg/vial 1 - Sterile vehicle - 17 mL/vial 5 - Sterile vehicle - 17 mL/vial 10 - Sterile vehicle - 17 mL/vial - Client Information Sheet
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1 Vial Kit Carton
- PRINCIPAL DISPLAY PANEL - 5 Vial Kit Carton
- PRINCIPAL DISPLAY PANEL - 10 Vial Kit Carton
-
INGREDIENTS AND APPEARANCE
PROHEART 6
moxidectin kitProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-3670 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-3670-1 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 VIAL, MULTI-DOSE 10 mL Part 2 1 VIAL, MULTI-DOSE 17 mL Part 1 of 2 PROHEART
moxidectin injectionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 3.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength TRISTEARIN (UNII: P6OCJ2551R) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141189 06/06/2001 Part 2 of 2 STERILE VEHICLE
inert injectionProduct Information Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 17 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141189 06/06/2001 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141189 06/06/2001 PROHEART 6
moxidectin kitProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-3671 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-3671-1 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 5 VIAL, MULTI-DOSE 88 mL Part 2 5 VIAL, MULTI-DOSE 85 mL Part 1 of 2 PROHEART
moxidectin injectionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 3.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength TRISTEARIN (UNII: P6OCJ2551R) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 17.6 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141189 06/06/2001 Part 2 of 2 STERILE VEHICLE
inert injectionProduct Information Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 17 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141189 06/06/2001 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141189 06/06/2001 PROHEART 6
moxidectin kitProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-3672 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-3672-1 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 VIAL, MULTI-DOSE 176 mL Part 2 10 VIAL, MULTI-DOSE 170 mL Part 1 of 2 PROHEART
moxidectin injectionProduct Information Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 3.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength TRISTEARIN (UNII: P6OCJ2551R) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 17.6 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141189 06/06/2001 Part 2 of 2 STERILE VEHICLE
inert injectionProduct Information Route of Administration SUBCUTANEOUS Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 17 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141189 06/06/2001 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141189 06/06/2001 Labeler - Zoetis Inc. (828851555)