RESCRIPTOR- delavirdine mesylate tablet
ViiV Healthcare Company
----------
PRESCRIBING INFORMATION
RESCRIPTOR
(delavirdine mesylate)
tablets
DESCRIPTION
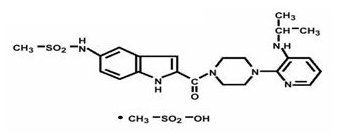
RESCRIPTOR tablets contain delavirdine mesylate, a synthetic non-nucleoside reverse transcriptase inhibitor (NNRTI) of the human immunodeficiency virus type 1 (HIV-1). The chemical name of delavirdine mesylate is piperazine, 1-[3-[(1-methyl-ethyl)amino]-2- pyridinyl]-4-[[5-[(methylsulfonyl)amino]-1H-indol-2-yl]carbonyl]-, monomethanesulfonate. Its molecular formula is C22H28N6O3S•CH4O3S, and its molecular weight is 552.68. The structural formula is:
Delavirdine mesylate is an odorless white-to-tan crystalline powder. The aqueous solubility of delavirdine free base at 23°C is 2,942 mcg/mL at pH 1.0, 295 mcg/mL at pH 2.0, and 0.81 mcg/mL at pH 7.4.
Each RESCRIPTOR tablet, for oral administration, contains 200 mg of delavirdine mesylate (henceforth referred to as delavirdine). Inactive ingredients consist of carnauba wax, colloidal silicon dioxide, croscarmellose sodium, lactose, magnesium stearate, and microcrystalline cellulose. In addition, the 200-mg tablet contains hypromellose and Opadry White YS-1-18202-A.
MICROBIOLOGY
Mechanism of Action:
Delavirdine is an NNRTI of HIV-1. Delavirdine binds directly to reverse transcriptase (RT) and blocks RNA-dependent and DNA-dependent DNA polymerase activities. Delavirdine does not compete with template:primer or deoxynucleoside triphosphates. HIV-2 RT and human cellular DNA polymerases α, γ, or δ are not inhibited by delavirdine. In addition, HIV-1 group O, a group of highly divergent strains that are uncommon in North America, may not be inhibited by delavirdine.
In Vitro HIV-1 Susceptibility: In vitro anti-HIV-1 activity of delavirdine was assessed by infecting cell lines of lymphoblastic and monocytic origin and peripheral blood lymphocytes with laboratory and clinical isolates of HIV-1. IC50 and IC90 values (50% and 90% inhibitory concentrations) for laboratory isolates (n = 5) ranged from 0.005 to 0.030 microM and 0.04 to 0.10 microM, respectively. Mean IC50 of clinical isolates (n = 74) was 0.038 microM (range: 0.001 to 0.69 microM); 73 of 74 clinical isolates had an IC50 ≤0.18 microM. The IC90 of 24 of these clinical isolates ranged from 0.05 to 0.10 microM. In drug combination studies of delavirdine with zidovudine, didanosine, zalcitabine, lamivudine, interferon-α, and protease inhibitors, additive to synergistic anti–HIV-1 activity was observed in cell culture. The relationship between the in vitro susceptibility of HIV-1 RT inhibitors and the inhibition of HIV replication in humans has not been established.
Drug Resistance: Phenotypic analyses of isolates from patients treated with RESCRIPTOR as monotherapy showed a 50- to 500-fold reduced susceptibility in 14 of 15 patients by Week 8 of therapy. Genotypic analysis of HIV-1 isolates from patients receiving RESCRIPTOR plus zidovudine combination therapy (n = 79) showed resistance-conferring mutations in all isolates by Week 24 of therapy. In patients treated with RESCRIPTOR, the mutations in RT occurred predominantly at amino acid positions 103 and less frequently at positions 181 and 236. In a separate study, an average of 86-fold increase in the zidovudine susceptibility of patient isolates (n = 24) was observed after 24 weeks of combination therapy with RESCRIPTOR and zidovudine. The clinical relevance of the phenotypic and the genotypic changes associated with therapy with RESCRIPTOR has not been established.
Cross-Resistance: RESCRIPTOR may confer cross-resistance to other NNRTIs when used alone or in combination. Mutations at positions 103 and/or 181 have been found in resistant virus during treatment with RESCRIPTOR and other NNRTIs. These mutations have been associated with cross-resistance among NNRTIs in vitro.
CLINICAL PHARMACOLOGY
Pharmacokinetics:
Absorption and Bioavailability: Delavirdine is rapidly absorbed following oral administration, with peak plasma concentrations occurring at approximately 1 hour. Following administration of delavirdine 400 mg 3 times daily (n = 67, HIV-1–infected patients), the mean ±SD steady-state peak plasma concentration (Cmax) was 35 ± 20 microM (range: 2 to 100 microM), systemic exposure (AUC) was 180 ± 100 microM per hour (range: 5 to 515 microM per hour), and trough concentration (Cmin) was 15 ± 10 microM (range: 0.1 to 45 microM). The single-dose bioavailability of delavirdine tablets relative to an oral solution was 85% ± 25% (n = 16, non-HIV–infected subjects). see DOSAGE AND ADMINISTRATION).
Delavirdine may be administered with or without food. In a multiple-dose, crossover study, delavirdine was administered every 8 hours with food or every 8 hours, 1 hour before or 2 hours after a meal (n = 13, HIV-1–infected patients). Patients remained on their typical diet throughout the study; meal content was not standardized. When multiple doses of delavirdine were administered with food, geometric mean Cmax was reduced by approximately 25%, but AUC and Cmin were not altered.
Distribution: Delavirdine is extensively bound (approximately 98%) to plasma proteins, primarily albumin. The percentage of delavirdine that is protein-bound is constant over a delavirdine concentration range of 0.5 to 196 microM. In 5 HIV-1–infected patients whose total daily dose of delavirdine ranged from 600 to 1,200 mg, cerebrospinal fluid concentrations of delavirdine averaged 0.4% ± 0.07% of the corresponding plasma delavirdine concentrations; this represents about 20% of the fraction not bound to plasma proteins. Steady-state delavirdine concentrations in saliva (n = 5, HIV-1–infected patients who received delavirdine 400 mg 3 times daily) and semen (n = 5 healthy volunteers who received delavirdine 300 mg 3 times daily) were about 6% and 2%, respectively, of the corresponding plasma delavirdine concentrations collected at the end of a dosing interval.
Metabolism and Elimination: Delavirdine is extensively converted to several inactive metabolites. Delavirdine is primarily metabolized by cytochrome P450 3A (CYP3A), but in vitro data suggest that delavirdine may also be metabolized by CYP2D6. The major metabolic pathways for delavirdine are N-desalkylation and pyridine hydroxylation. Delavirdine exhibits nonlinear steady-state elimination pharmacokinetics, with apparent oral clearance decreasing by about 22-fold as the total daily dose of delavirdine increases from 60 to 1,200 mg/day. In a study of 14C-delavirdine in 6 healthy volunteers who received multiple doses of delavirdine tablets 300 mg 3 times daily, approximately 44% of the radiolabeled dose was recovered in feces, and approximately 51% of the dose was excreted in urine. Less than 5% of the dose was recovered unchanged in urine. The parent plasma half-life of delavirdine increases with dose; mean half-life following 400 mg 3 times daily is 5.8 hours, with a range of 2 to 11 hours.
In vitro and in vivo studies have shown that delavirdine reduces CYP3A activity and inhibits its own metabolism. In vitro studies have also shown that delavirdine reduces CYP2C9, CYP2D6, and CYP2C19 activity. Inhibition of hepatic CYP3A activity by delavirdine is reversible within 1 week after discontinuation of drug.
Special Populations:
Hepatic or Renal Impairment: The pharmacokinetics of delavirdine in patients with hepatic or renal impairment have not been investigated (see PRECAUTIONS).
Age: The pharmacokinetics of delavirdine have not been adequately studied in patients younger than 16 years or older than 65 years.
Gender: Data from population pharmacokinetics suggest that the plasma concentrations of delavirdine tend to be higher in females than in males. However, this difference is not considered to be clinically significant.
Race: No significant differences in the mean trough delavirdine concentrations were observed between different racial or ethnic groups.
Drug Interactions:
(See also PRECAUTIONS: Drug Interactions.)
Specific drug interaction studies were performed with delavirdine and a number of drugs. Table 1 summarizes the effects of delavirdine on the geometric mean AUC, Cmax, and Cmin of coadministered drugs. Table 2 shows the effects of coadministered drugs on the geometric mean AUC, Cmax, and Cmin of delavirdine.
For information regarding clinical recommendations, see CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS: Drug Interactions.
| ↑ Indicates increase. ↓ Indicates decrease. ↔ Indicates no significant change. - Indicates no data available. a Relative to indinavir 800 mg t.i.d. without RESCRIPTOR. b Plasma concentrations of the nelfinavir active metabolite (nelfinavir hydroxy-t-butylamide) were significantly reduced by delavirdine, which is more than compensated for by increased nelfinavir concentration. c Saquinavir soft gel capsule 1,000 mg t.i.d. plus RESCRIPTOR 400 mg t.i.d. relative to saquinavir soft gel capsule 1,200 mg t.i.d. without RESCRIPTOR. d RESCRIPTOR taken with didanosine (buffered tablets) relative to doses of RESCRIPTOR and didanosine (buffered tablets) separated by at least 1 hour. |
||||||
|
Coadministered Drug |
Dose of Coadministered Drug |
Dose of RESCRIPTOR |
n |
% Change in Pharmacokinetic Parameters of Coadministered Drug (90% CI) |
||
|
Cmax |
AUC |
Cmin |
||||
|
HIV-Protease Inhibitors |
||||||
|
Indinavir |
400 mg t.i.d. for 7 days |
400 mg t.i.d. for 7 days |
28 |
↓36a (↓52 to ↓14) |
↔ a |
↑118 a (↑16 to ↑312) |
|
600 mg t.i.d. for 7 days |
400 mg t.i.d. for 7 days |
28 |
↔ |
↑53 a (↑7 to ↑120) |
↑298 a (↑104 to ↑678) |
|
|
Nelfinavirb |
750 mg t.i.d. for 14 days |
400 mg t.i.d. for 7 days |
12 |
↑88 (↑66 to ↑113) |
↑107 (↑83 to ↑135) |
↑136 (↑103 to ↑175) |
|
Saquinavir |
Soft gel capsule 1,000 mg t.i.d. for 28 days |
400 mg t.i.d. for 28 days |
20 |
↑98c (↑4 to ↑277) |
↑121c (↑14 to ↑340) |
↑199c (↑37 to ↑553) |
|
Nucleoside Reverse Transcriptase Inhibitors |
||||||
|
Didanosine (buffered tablets) |
125 or 250 mg b.i.d. for 28 days |
400 mg t.i.d. for 28 days |
9 |
↓20d (↓44 to ↑15) |
↓21d (↓40 to ↑5) |
- |
|
Zidovudine |
200 mg t.i.d. for >38 days |
100 mg q.i.d. to 400 mg t.i.d. for 8 to 10 days |
34 |
↔ |
↔ |
- |
|
Anti-infective Agents |
||||||
|
Clarithromycin |
500 mg b.i.d. for 15 days |
300 mg t.i.d. for 30 days |
6 |
- |
↑100 |
- |
|
Rifabutin |
300 mg q.d. for 15 to 99 days |
400 to 1,000 mg t.i.d. for 45 to 129 days |
5 |
↑128 (↑71 to ↑203) |
↑230 (↑119 to ↑396) |
↑452 (↑246 to ↑781) |
|
Coadministered Drug |
Dose of Coadministered Drug |
Dose of RESCRIPTOR |
n |
% Change in Delavirdine Pharmacokinetic Parameters (90% CI) |
||
|
Cmax |
AUC |
Cmin |
||||
|
HIV-Protease Inhibitors |
||||||
|
Indinavir |
400 or 600 mg t.i.d. for 7 days |
400 mg t.i.d. for 7 days |
81 |
No apparent changes based on a comparison to historical data |
||
|
Nelfinavir |
750 mg t.i.d. for 7 days |
400 mg t.i.d. for 14 days |
7 |
↓27 (↓49 to ↑4) |
↓31 (↓57 to ↑10) |
↓33 (↓70 to ↑49) |
|
Saquinavir |
Soft gel capsule 1,000 mg t.i.d. for 28 days |
400 mg t.i.d. for 7 to 28 days |
23 |
No apparent changes based on a comparison to historical data |
||
|
Nucleoside Reverse Transcriptase Inhibitors |
||||||
|
Didanosine (buffered tablets) |
125 or 200 mg b.i.d. for 28 days |
400 mg t.i.d. for 28 days |
9 |
↓32a (↓48 to↓11) |
↓19 a (↓37 to ↑6) |
↔a |
|
Zidovudine |
200 mg t.i.d. for ≥7 days |
400 mg t.i.d. for 7 to 14 days |
42 |
No apparent changes based on a comparison to historical data |
||
|
Anti-infective Agents |
||||||
|
Clarithromycin |
500 mg b.i.d. for 15 days |
300 mg t.i.d. for 30 days |
6 |
↔ |
↔ |
↔ |
|
Fluconazole |
400 mg q.d. for 15 days |
300 mg t.i.d. for 30 days |
8 |
↔ |
↔ |
↔ |
|
Ketoconazole |
Various |
200 to 400 mg t.i.d. |
26 |
- |
- |
↑50b |
|
Rifabutin |
300 mg q.d. for 14 days |
400 mg t.i.d. for 28 days |
7 |
↓72 (↓61 to ↓80) |
↓82 (↓74 to ↓88) |
↓94 (↓90 to ↓96) |
|
Rifampin |
600 mg q.d. for 15 days |
400 mg t.i.d. for 30 days |
7 |
↓90 (↓94 to ↓83) |
↓97 (↓98 to ↓95) |
↓100 |
|
Sulfamethoxazole or Trimethoprim & Sulfamethoxazole |
Various |
200 to 400 mg t.i.d. |
311 |
- |
- |
↔b |
|
Other |
||||||
|
Antacid (MAALOX TC) |
20 mL |
300 mg single dose |
12 |
↓52 (↓68 to ↓29) |
↓44 (↓58 to ↓27) |
- |
|
Fluoxetine |
Various |
200 to 400 mg t.i.d. |
36 |
- |
- |
↑50b |
|
Phenytoin, Phenobarbital, Carbamazepine |
Various |
300 to 400 mg t.i.d. |
8 |
- |
- |
↓90b |
↑ Indicates increase.
↓ Indicates decrease.
↔ Indicates no significant change.
- Indicates no data available.
a RESCRIPTOR taken with didanosine (buffered tablets) relative to doses of RESCRIPTOR and didanosine (buffered tablets) separated by at least 1 hour.
b Population pharmacokinetic data from efficacy studies.
INDICATIONS AND USAGE
RESCRIPTOR tablets are indicated for the treatment of HIV-1 infection in combination with at least 2 other active antiretroviral agents when therapy is warranted.
The following should be considered before initiating therapy with RESCRIPTOR in treatment-naive patients. There are insufficient data directly comparing antiretroviral regimens containing RESCRIPTOR with currently preferred 3-drug regimens for initial treatment of HIV. In studies comparing regimens consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs) (currently considered suboptimal) to RESCRIPTOR plus 2 NRTIs, the proportion of patients receiving the regimen containing RESCRIPTOR who achieved and sustained an HIV-1 RNA level <400 copies/mL over 1 year of therapy was relatively low (see DESCRIPTION OF CLINICAL STUDIES).
Resistant virus emerges rapidly when RESCRIPTOR is administered as monotherapy. Therefore, RESCRIPTOR should always be administered in combination with other antiretroviral agents.
DESCRIPTION OF CLINICAL STUDIES
For clinical Studies 21 Part II and 13C described below, efficacy was evaluated by the percentage of patients with a plasma HIV-1 RNA level <400 copies/mL through Week 52 as measured by the Roche Amplicor HIV-1 Monitor (standard assay). An intent-to-treat analysis was performed where only subjects who achieved confirmed suppression and sustained it through Week 52 are regarded as responders. All other subjects (including never suppressed, discontinued, and those who rebounded after initial suppression of <400 copies/mL) are considered failures at Week 52. Results of an interim analysis of efficacy conducted for studies 21 Part II and 13C by independent Data and Safety Monitoring Boards (DSMBs) revealed that the triple-therapy arms in both studies produced significantly greater antiviral benefit than the dual-therapy arms, and early termination of the studies was recommended.
Study 21 Part II:
Study 21 Part II was a double‑blind, randomized, placebo‑controlled trial comparing treatment with RESCRIPTOR (400 mg 3 times daily, zidovudine 200 mg 3 times daily, and lamivudine 150 mg twice daily versus RESCRIPTOR 400 mg 3 times daily and zidovudine 200 mg 3 times daily versus zidovudine 200 mg 3 times daily and lamivudine 150 mg twice daily in 373 HIV-1–infected patients (mean age 35 years [range: 17 to 67], 87% male, and 60% Caucasian) who were antiretroviral treatment naive (84%) or had limited nucleoside experience (16%). Mean baseline CD4+ cell count was 359 cells/mm3 and mean baseline plasma HIV‑1 RNA was 4.4 log10 copies/mL.
Results showed that the mean increases from baseline in CD4 cell counts at 52 weeks were 111 cells/mL for RESCRIPTOR + zidovudine + lamivudine, 27 cells/mL for RESCRIPTOR + zidovudine, and 74 cells/mL for zidovudine + lamivudine.
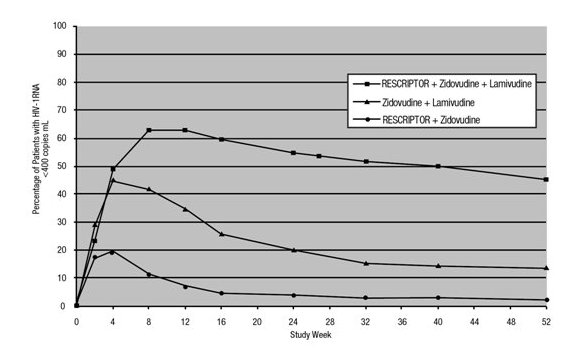
The results of the intent-to-treat analysis of the percentage of patients with a plasma HIV-1 RNA level <400 copies/mL are presented in Figure 1. HIV-1 RNA status and reasons for discontinuation of randomized treatment at 52 weeks are summarized in Table 3. Subjects who were never suppressed before discontinuation were placed in the discontinuation category.
Figure 1. Percentage of Patients with HIV-1 RNA Below 400 copies/mL
Standard PCR Assay
Protocol 21 Part II: Intent-to–Treat Analysis
| a Corresponds to rates at Week 52 in proportion curve. b Virologic failures at or before Week 52. c Considered to be treatment failure in the analysis. d Includes discontinuations due to consent withdrawn, loss to follow-up, protocol violations, non-compliance, pregnancy, never treated, and other reasons. |
|||
|
|
Zidovudine + Lamivudine
|
RESCRIPTOR + Zidovudine
|
RESCRIPTOR + Zidovudine + Lamivudine
|
|
HIV-1 RNA <400 copies/mLa |
14 |
2 |
45 |
|
HIV-1 RNA ≥400 copies/mLb,c |
64 |
52 |
31 |
|
Discontinued due to adverse eventsc |
8 |
13 |
10 |
|
Discontinued due to other reasonsc,d |
14 |
33 |
14 |
Study 13C: Study 13C was a double‑blind, randomized, placebo‑controlled trial comparing treatment with RESCRIPTOR 400 mg 3 times daily, zidovudine 200 mg 3 times daily or 300 mg twice daily, and either didanosine 200 mg twice daily, zalcitabine 0.75 mg 3 times daily, or lamivudine 150 mg twice daily versus zidovudine 200 mg 3 times daily or 300 mg twice daily and either didanosine 200 mg twice daily, zalcitabine 0.75 mg 3 times daily, or lamivudine 150 mg twice daily in 345 HIV-1–infected patients (mean age 35.8 years [range: 18 to 72], 66% male, and 63% Caucasian) who were antiretroviral treatment naive (63%) or had limited antiretroviral experience (37%). Mean baseline CD4+ cell count was 210 cells/mm3 and mean baseline plasma HIV‑1 RNA was 4.9 log10 copies/mL.
Results showed that the mean increases from baseline in CD4+ cell counts at 54 weeks were 102 cells/mL for RESCRIPTOR + zidovudine + didanosine or zalcitabine or lamivudine, and 56 cells/mL for zidovudine + didanosine or zalcitabine or lamivudine.
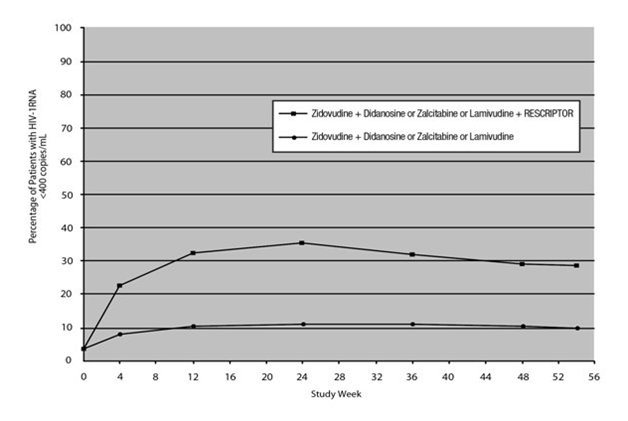
The results of the intent‑to‑treat analysis of the percentage of patients with a plasma HIV‑1 RNA level 400 copies/mL are presented in Figure 2. HIV‑1 RNA status and reasons for discontinuation of randomized treatment at 54 weeks are summarized in Table 4. Subjects who were never suppressed before discontinuation were placed in the discontinuation category.
Figure 2. Percentage of Patients with HIV-1 RNA Below 400 copies/mL
Standard PCR Assay
Protocol 13C: Intent-to–Treat Analysis
| a Corresponds to rates at Week 54 in proportion curve. b Virologic failures at or before Week 54. c Considered to be treatment failure in the analysis. d Includes discontinuations due to consent withdrawn, loss to follow-up, protocol violations, non-compliance, pregnancy, never treated, and other reasons. |
||||||
|
Outcome |
Zidovudine + Didanosine or Zalcitabine or Lamivudine
|
Zidovudine + Didanosine or Zalcitabine or Lamivudine + RESCRIPTOR
|
||||
|
HIV‑1 RNA <400 copies/mLa |
10 |
29 |
||||
|
HIV‑1 RNA ≥400 copies/mLb,c |
69 |
42 |
||||
|
Discontinued due to adverse eventsc |
7 |
12 |
||||
|
Discontinued due to other reasonsc,d |
14 |
17 |
||||
Results from several smaller supportive studies evaluating the use of RESCRIPTOR in treatment-naive patients suggest that it may have activity when used in combination with protease inhibitors and NRTIs in 3- or 4-drug combinations.
CONTRAINDICATIONS
RESCRIPTOR tablets are contraindicated in patients with known hypersensitivity to any of its ingredients. Coadministration of RESCRIPTOR is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events. These drugs are listed in Table 5. Also, see PRECAUTIONS, Table 6, Drugs That Should Not Be Coadministered with RESCRIPTOR.
|
Drug Class |
Drugs within Class That Are Contraindicated with RESCRIPTOR |
|
Antihistamines |
Astemizole, terfenadine |
|
Ergot derivatives |
Dihydroergotamine, ergonovine, ergotamine, methylergonovine |
|
GI motility agent |
Cisapride |
|
Neuroleptic |
Pimozide |
|
Sedative/hypnotics |
Alprazolam, midazolam, triazolam |
WARNINGS
ALERT: Find out about medicines that should NOT be taken with RESCRIPTOR. This statement is included on the product’s bottle label.
Drug Interactions:
Because delavirdine may inhibit the metabolism of many different drugs (e.g., antiarrhythmics, calcium channel blockers, sedative hypnotics, and others), serious and/or life-threatening drug interactions could result from inappropriate coadministration of some drugs with delavirdine. In addition, some drugs may markedly reduce delavirdine plasma concentrations, resulting in suboptimal antiviral activity and subsequent emergence of drug resistance. All prescribers should become familiar with the following tables in this package insert: Table 5, Drugs That Are Contraindicated with RESCRIPTOR; Table 6, Drugs That Should Not Be Coadministered with RESCRIPTOR; and Table 7, Established and Other Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interaction. Additional details on drug interactions can be found in Tables 1 and 2 under the CLINICAL PHARMACOLOGY section.
Concomitant use of lovastatin or simvastatin with RESCRIPTOR is not recommended. Caution should be exercised if RESCRIPTOR is used concurrently with other HMG-CoA reductase inhibitors that are also metabolized by the CYP3A4 pathway (e.g., atorvastatin or cerivastatin). The risk of myopathy including rhabdomyolysis may be increased when RESCRIPTOR is used in combination with these drugs.
Particular caution should be used when prescribing sildenafil in patients receiving RESCRIPTOR. Coadministration of sildenafil with RESCRIPTOR is expected to substantially increase sildenafil concentrations and may result in an increase in sildenafil-associated adverse events, including hypotension, visual changes, and priapism (see PRECAUTIONS: Drug Interactions and Information for Patients, and the complete prescribing information for sildenafil).
Concomitant use of St. John’s wort (Hypericum perforatum) or St. John’s wort-containing products and RESCRIPTOR is not recommended. Coadministration of St. John’s wort with NNRTIs, including RESCRIPTOR, is expected to substantially decrease NNRTI concentrations and may result in suboptimal levels of RESCRIPTOR and lead to loss of virologic response and possible resistance to RESCRIPTOR or to the class of NNRTIs.
PRECAUTIONS
General:
Delavirdine is metabolized primarily by the liver. Therefore, caution should be exercised when administering RESCRIPTOR tablets to patients with impaired hepatic function.
Immune Reconstitution Syndrome:
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including RESCRIPTOR. During the initial phase of the combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
Resistance/Cross-Resistance:
NNRTIs, when used alone or in combination, may confer cross-resistance to other NNRTIs.
Fat Redistribution:
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Skin Rash:
Severe rash, including rare cases of erythema multiforme and Stevens-Johnson syndrome, has been reported in patients receiving RESCRIPTOR. Erythema multiforme and Stevens-Johnson syndrome were rarely seen in clinical trials and resolved after withdrawal of RESCRIPTOR. Any patient experiencing severe rash or rash accompanied by symptoms such as fever, blistering, oral lesions, conjunctivitis, swelling, and muscle or joint aches should discontinue RESCRIPTOR and consult a physician. Two cases of Stevens-Johnson syndrome have been reported through postmarketing surveillance out of a total of 339 surveillance reports.
In Studies 21 Part II and 13C (see DESCRIPTION OF CLINICAL STUDIES), rash (including maculopapular rash) was reported in more patients who were treated with RESCRIPTOR 400 mg 3 times daily (35% and 32%, respectively) than in those who were not treated with RESCRIPTOR (21% and 16%, respectively). The highest intensity of rash reported in these studies was severe (Grade 3), which was observed in approximately 4% of patients treated with RESCRIPTOR in each study and in none of the patients who were not treated with RESCRIPTOR. Also in Studies 21 Part II and 13C, discontinuations due to rash were reported in more patients who received RESCRIPTOR 400 mg 3 times daily (3% and 4%, respectively) than in those who did not receive RESCRIPTOR (0% and 1%, respectively).
In most cases, the duration of the rash was less than 2 weeks and did not require dose reduction or discontinuation of RESCRIPTOR. Most patients were able to resume therapy after rechallenge with RESCRIPTOR following a treatment interruption due to rash. The distribution of the rash was mainly on the upper body and proximal arms, with decreasing intensity of the lesions on the neck and face, and progressively less on the rest of the trunk and limbs.
Occurrence of a delavirdine-associated rash after 1 month is uncommon. Symptomatic relief has been obtained using diphenhydramine hydrochloride, hydroxyzine hydrochloride, and/or topical corticosteroids.
Information for Patients:
A statement to patients and healthcare providers is included on the product’s bottle label: ALERT: Find out about medicines that should NOT be taken with RESCRIPTOR. A patient package insert (PPI) for RESCRIPTOR is available for patient information.
Patients should be informed that RESCRIPTOR is not a cure for HIV‑1 infection and that patients may continue to experience illnesses associated with HIV‑1 infection, including opportunistic infections. Patients should be advised to remain under the care of a physician while taking RESCRIPTOR.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
- •
- Do not share needles or other injection equipment.
- •
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- •
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- •
- Do not breastfeed. We do not know if RESCRIPTOR can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
Patients should be instructed that the major toxicity of RESCRIPTOR is rash and should be advised to promptly notify their physician should rash occur. The majority of rashes associated with RESCRIPTOR occur within 1 to 3 weeks after initiating treatment with RESCRIPTOR. The rash normally resolves in 3 to 14 days and may be treated symptomatically while therapy with RESCRIPTOR is continued. Any patient experiencing severe rash or rash accompanied by symptoms such as fever, blistering, oral lesions, conjunctivitis, swelling, and muscle or joint aches should discontinue medication and consult a physician.
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
Patients should be informed to take RESCRIPTOR every day as prescribed. Patients should not alter the dose of RESCRIPTOR without consulting their doctor. If a dose is missed, patients should take the next dose as soon as possible. However, if a dose is skipped, the patient should not double the next dose.
Patients with achlorhydria should take RESCRIPTOR with an acidic beverage (e.g., orange or cranberry juice). However, the effect of an acidic beverage on the absorption of delavirdine in patients with achlorhydria has not been investigated.
Patients taking both RESCRIPTOR and antacids should be advised to take them at least 1 hour apart.
Because RESCRIPTOR may interact with certain drugs, patients should be advised to report to their doctor the use of any prescription, nonprescription medication, or herbal products, particularly St. John’s wort.
Patients receiving sildenafil and RESCRIPTOR should be advised that they may be at an increased risk of sildenafil‑associated adverse events, including hypotension, visual changes, and prolonged penile erection, and should promptly report any symptoms to their doctor.
Drug Interactions:
(See also CONTRAINDICATIONS, WARNINGS, and CLINICAL PHARMACOLOGY: Drug Interactions.)
Delavirdine is an inhibitor of CYP3A isoform and other CYP isoforms to a lesser extent including CYP2C9, CYP2D6, and CYP2C19. Coadministration of RESCRIPTOR and drugs primarily metabolized by CYP3A (e.g., HMG-CoA reductase inhibitors and sildenafil) may result in increased plasma concentrations of the coadministered drug that could increase or prolong both its therapeutic or adverse effects.
Delavirdine is metabolized primarily by CYP3A, but in vitro data suggest that delavirdine may also be metabolized by CYP2D6. Coadministration of RESCRIPTOR and drugs that induce CYP3A, such as rifampin, may decrease delavirdine plasma concentrations and reduce its therapeutic effect. Coadministration of RESCRIPTOR and drugs that inhibit CYP3A may increase delavirdine plasma concentrations. (See Table 6, Drugs That Should Not Be Coadministered with RESCRIPTOR, and Table 7, Established and Other Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interaction.)
| a See CLINICAL PHARMACOLOGY for magnitude of interaction, Tables 1 and 2. | |
|
Drug Class: Drug Name |
Clinical Comment |
|
Anticonvulsant agents: Phenytoin, phenobarbital, carbamazepine |
May lead to loss of virologic response and possible resistance to RESCRIPTOR or to the class of NNRTIs. |
|
Antihistamines: Astemizole, terfenadine |
CONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
|
Antimycobacterials: Rifabutin,a rifampin a |
May lead to loss of virologic response and possible resistance to RESCRIPTOR or to the class of NNRTIs or other coadministered antiviral agents. |
|
Ergot Derivatives: Dihydroergotamine, ergonovine, ergotamine, methylergonovine |
CONTRAINDICATED due to potential for serious and/or life-threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues. |
|
GI motility agent: Cisapride |
CONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
|
Herbal Products: St. John’s wort |
May lead to loss of virologic response and possible resistance to RESCRIPTOR or to the class of NNRTIs. |
|
HMG-CoA reductase inhibitors: Lovastatin, simvastatin |
Potential for serious reactions such as risk of myopathy including rhabdomyolysis. |
|
Neuroleptic: Pimozide |
CONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
|
Sedative/hypnotics: Alprazolam, midazolam, triazolam |
CONTRAINDICATED due to potential for serious and/or life-threatening reactions such as prolonged or increased sedation or respiratory depression. |
| ↑ Indicates increase. ↓ Indicates decrease. a The interaction between RESCRIPTOR and the drug was evaluated in a clinical study. All other drug interactions shown are predicted. |
||||
|
Concomitant Drug Class: Drug Name |
Effect on Concentration of Delavirdine or Concomitant Drug |
Clinical Comment |
||
|
HIV-Antiviral Agents: Nucleoside Reverse Transcriptase Inhibitor |
||||
|
Didanosinea |
↓Delavirdine ↓Didanosine |
Administration of didanosine (buffered tablets) and RESCRIPTOR should be separated by at least 1 hour. |
||
|
HIV-Antiviral Agents: Non-nucleoside Reverse Transcriptase Inhibitors |
||||
|
NNRTI |
↔Delavirdine ↑NNRTI |
Combining NNRTIs has not been shown to be beneficial. RESCRIPTOR should not be coadministered with another NNRTI. |
||
|
HIV-Antiviral Agents: Protease Inhibitors |
||||
|
Indinavira |
↑Indinavir |
A dose reduction of indinavir to 600 mg 3 times daily should be considered when RESCRIPTOR and indinavir are coadministered. |
||
|
Lopinavir/Ritonavir |
↑Lopinavir ↑Ritonavir |
Appropriate doses of this combination with respect to safety, efficacy, and pharmacokinetics have not been established. |
||
|
Nelfinavira |
↑Nelfinavir ↓Delavirdine |
Appropriate doses of this combination with respect to safety, efficacy, and pharmacokinetics have not been established (see CLINICAL PHARMACOLOGY: Tables 1 and 2). |
||
|
Ritonavir |
↑Ritonavir |
Appropriate doses of this combination with respect to safety, efficacy, and pharmacokinetics have not been established. |
||
|
Saquinavira |
↑Saquinavir |
A dose reduction of saquinavir (soft gelatin capsules) may be considered when RESCRIPTOR and saquinavir are coadministered (see CLINICAL PHARMACOLOGY: Table 1). Appropriate doses with respect to safety, efficacy, and pharmacokinetics have not been established. |
||
|
HIV-Antiviral Agents: CCR5 Inhibitor |
||||
|
Maraviroc |
↑Maraviroc |
Concomitant use of RESCRIPTOR and maraviroc has not been studied. However, RESCRIPTOR is a potent CYP3A4 inhibitor and the maraviroc dose should be reduced during coadministration. Refer to the full prescribing information for maraviroc (SELZENTRY) for dosing recommendations. |
||
|
Other Agents |
||||
|
Acid blockers: Antacidsa |
↓Delavirdine |
Doses of an antacid and RESCRIPTOR should be separated by at least 1 hour, because the absorption of delavirdine is reduced when coadministered with antacids. |
||
|
Histamine H2-receptor antagonists: Cimetidine, famotidine, nizatidine, ranitidine |
↓Delavirdine |
These agents increase gastric pH and may reduce the absorption of delavirdine. Although the effect of these drugs on delavirdine absorption has not been evaluated, chronic use of these drugs with RESCRIPTOR is not recommended. |
||
|
Proton pump inhibitors: Omeprazole, lansoprazole |
↓Delavirdine |
These agents increase gastric pH and may reduce the absorption of delavirdine. Although the effect of these drugs on delavirdine absorption has not been evaluated, chronic use of these drugs with RESCRIPTOR is not recommended. |
||
|
Amphetamines |
↑Amphetamines |
Use with caution. |
||
|
Antidepressant: Trazodone |
↑Trazodone |
Concomitant use of trazodone and RESCRIPTOR may increase plasma concentrations of trazodone. Adverse events of nausea, dizziness, hypotension, and syncope have been observed following coadministration of trazodone and ritonavir. If trazodone is used with a CYP3A4 inhibitor such as RESCRIPTOR, the combination should be used with caution and a lower dose of trazodone should be considered. |
||
|
Antiarrhythmics: Bepridil |
↑Antiarrhythmics |
Use with caution. Increased bepridil exposure may be associated with life‑threatening reactions such as cardiac arrhythmias. |
||
|
Amiodarone, lidocaine (systemic), quinidine, flecainide, propafenone |
Caution is warranted and therapeutic concentration monitoring is recommended, if available, for antiarrhythmics when coadministered with RESCRIPTOR. |
|||
|
Anticoagulant: Warfarin |
↑Warfarin |
It is recommended that INR (international normalized ratio) be monitored. |
||
|
Anti-infective: Clarithromycina |
↑Clarithromycin |
When coadministered with RESCRIPTOR, clarithromycin should be adjusted in patients with impaired renal function:
|
||
|
Calcium channel blockers: Amlodipine, diltiazem, felodipine, isradipine, nifedipine, nicardipine, nimodipine, nisoldipine, verapamil |
↑Calcium channel blockers |
Caution is warranted and clinical monitoring of patients is recommended. |
||
|
Corticosteroid: Dexamethasone |
↓Delavirdine |
Use with caution. RESCRIPTOR may be less effective due to decreased delavirdine plasma concentrations in patients taking these agents concomitantly. |
||
|
Erectile dysfunction agents: Sildenafil |
↑Sildenafil |
Sildenafil should not exceed a maximum single dose of 25 mg in a 48‑hour period. |
||
|
HMG-CoA reductase inhibitors: Atorvastatin, cerivastatin, fluvastatin |
↑Atorvastatin ↑Cerivastatin ↑Fluvastatin |
Use lowest possible dose of atorvastatin or cerivastatin, or fluvastatin with careful monitoring, or consider other HMG-CoA reductase inhibitors such as pravastatin in combination with RESCRIPTOR. |
||
|
Immunosuppressants: Cyclosporine, tacrolimus, rapamycin |
↑Immunosuppressants |
Therapeutic concentration monitoring is recommended for immunosuppressant agents when coadministered with RESCRIPTOR. |
||
|
Inhaled/nasal steroid: Fluticasone |
↑Fluticasone |
Concomitant use of fluticasone and RESCRIPTOR may increase plasma concentrations of fluticasone. Use with caution. Consider alternatives to fluticasone, particularly for long-term use. |
||
|
Narcotic analgesic: Methadone |
↑Methadone |
Dosage of methadone may need to be decreased when coadministered with RESCRIPTOR. |
||
|
Oral contraceptives: Ethinyl estradiol |
↑Ethinyl estradiol |
Concentrations of ethinyl estradiol may increase. However, the clinical significance is unknown. |
||
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Delavirdine was negative in a battery of genetic toxicology tests which included an Ames assay, an in vitro rat hepatocyte unscheduled DNA synthesis assay, an in vitro chromosome aberration assay in human peripheral lymphocytes, an in vitro mutation assay in Chinese hamster ovary cells, and an in vivo micronucleus test in mice.
Lifetime carcinogenicity studies were conducted in rats at doses of 10, 32, and 100 mg/kg/day and in mice at doses of 62.5, 250, and 500 mg/kg/day for males and 62.5, 125, and 250 mg/kg/day for females. In rats, delavirdine was noncarcinogenic at maximally tolerated doses that produced exposures (AUC) up to 12 (male rats) and 9 (female rats) times human exposure at the recommended clinical dose. In mice, delavirdine produced significant increases in the incidence of hepatocellular adenoma/adenocarcinoma in both males and females, hepatocellular adenoma in females, and mesenchymal urinary bladder tumors in males. The systemic drug exposures (AUC) in female mice were 0.5- to 3-fold and in male mice 0.2- to 4-fold of those in humans at the recommended clinical dose. Given the lack of genotoxic activity of delavirdine, the relevance of urinary bladder and hepatocellular neoplasm in delavirdine-treated mice to humans is not known.
Delavirdine at doses of 20, 100, and 200 mg/kg/day did not cause impairment of fertility in rats when males were treated for 70 days and females were treated for 14 days prior to mating.
Pregnancy:
Delavirdine has been shown to be teratogenic in rats. Delavirdine caused ventricular septal defects in rats at doses of 50, 100, and 200 mg/kg/day when administered during the period of organogenesis. The lowest dose of delavirdine that caused malformations produced systemic exposures in pregnant rats equal to or lower than the expected human exposure to RESCRIPTOR (Cmin 15 microM) at the recommended dose. Exposure in rats approximately 5-fold higher than the expected human exposure resulted in marked maternal toxicity, embryotoxicity, fetal developmental delay, and reduced pup survival. Additionally, reduced pup survival on postpartum Day 0 occurred at an exposure (mean Cmin) approximately equal to the expected human exposure. Delavirdine was excreted in the milk of lactating rats at a concentration 3 to 5 times that of rat plasma.
Delavirdine at doses of 200 and 400 mg/kg/day administered during the period of organogenesis caused maternal toxicity, embryotoxicity, and abortions in rabbits. The lowest dose of delavirdine that resulted in these toxic effects produced systemic exposures in pregnant rabbits approximately 6-fold higher than the expected human exposure to RESCRIPTOR (Cmin 15 microM) at the recommended dose. The no-observed-adverse-effect dose in the pregnant rabbit was 100 mg/kg/day. Various malformations were observed at this dose, but the incidence of such malformations was not statistically significantly different from that observed in the control group. Systemic exposures in pregnant rabbits at a dose of 100 mg/kg/day were lower than those expected in humans at the recommended clinical dose. Malformations were not apparent at 200 and 400 mg/kg/day; however, only a limited number of fetuses were available for examination as a result of maternal and embryo death.
No adequate and well-controlled studies in pregnant women have been conducted. RESCRIPTOR should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Of 9 pregnancies reported in premarketing clinical studies and postmarketing experience, a total of 10 infants were born (including 1 set of twins). Eight of the infants were born healthy. One infant was born HIV-positive but was otherwise healthy and with no congenital abnormalities detected, and 1 infant was born prematurely (34 to 35 weeks) with a small muscular ventricular septal defect that spontaneously resolved. The patient received approximately 6 weeks of treatment with delavirdine and zidovudine early in the course of the pregnancy.
Antiretroviral Pregnancy Registry: To monitor maternal-fetal outcomes of pregnant women exposed to RESCRIPTOR and other antiretroviral agents, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling (800) 258-4263.
Nursing Mothers:
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Because of both the potential for HIV transmission and any possible adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving RESCRIPTOR.
Pediatric Use:
Safety and effectiveness of delavirdine in combination with other antiretroviral agents have not been established in HIV-1–infected individuals younger than 16 years of age.
Geriatric Use:
Clinical studies of RESCRIPTOR did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, caution should be taken when dosing RESCRIPTOR in elderly patients due to the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The safety of RESCRIPTOR tablets alone and in combination with other therapies has been studied in approximately 6,000 patients receiving RESCRIPTOR. The majority of adverse events were of mild or moderate (i.e., ACTG Grade 1 or 2) intensity. The most frequently reported drug-related adverse event (i.e., events considered by the investigator to be related to the blinded study medication or events with an unknown or missing causal relationship to the blinded medication) among patients receiving RESCRIPTOR was skin rash (see Table 8 and PRECAUTIONS: Skin Rash).
| a Includes events reported regardless of causality. b ACTG Toxicity Grading System; includes events reported as “rash,” “maculopapular rash,” and “urticaria.” |
|||
|
Percent of Patients with: |
Description of Rash Gradeb |
RESCRIPTOR 400 mg t.i.d. (n = 412) |
Control Group Patients (n = 295) |
|
Grade 1 rash |
Erythema, pruritus |
69 (16.7%) |
35 (11.9%) |
|
Grade 2 rash |
Diffuse maculopapular rash, dry desquamation |
59 (14.3%) |
17 (5.8%) |
|
Grade 3 rash |
Vesiculation, moist desquamation, ulceration |
18 (4.4%) |
0 (0.0%) |
|
Grade 4 rash |
Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, necrosis requiring surgery, exfoliative dermatitis |
0 (0.0%) |
0 (0.0%) |
|
Rash of any grade |
146 (35.4%) |
52 (17.6%) |
|
|
Treatment discontinuation as a result of rash |
13 (3.2%) |
1 (0.3%) |
|
Adverse events of moderate to severe intensity reported by at least 5% of evaluable patients in any treatment group in the pivotal trials, which includes patients receiving RESCRIPTOR in combination with zidovudine and/or lamivudine in Study 21 Part II for up to 98 weeks and in combination with zidovudine and either lamivudine, didanosine, or zalcitabine in Study 13C for up to 72 weeks are summarized in Table 9.
| a Evaluable patients in Study 21 Part II were those who received at least 1 dose of study medication and returned for at least 1 clinic study visit. Evaluable patients in Study 13C were those who received at least 1 dose of study medication. | |||||||||
|
Study 21 Part II |
Study 13C |
||||||||
|
Zidovudine + Lamivudine (n = 123) |
400 mg t.i.d. RESCRIPTOR + Zidovudine (n = 123) |
400 mg t.i.d. RESCRIPTOR + Zidovudine + Lamivudine (n = 119) |
Zidovudine + Didanosine, Zalcitabine, or Lamivudine (n = 172) |
400 mg t.i.d. RESCRIPTOR + Zidovudine + Didanosine, Zalcitabine, or Lamivudine (n = 170) |
|||||
|
Adverse Events |
% of pts. (n) |
% of pts. (n) |
% of pts. (n) |
% of pts. (n) |
% of pts. (n) |
||||
|
Body as a Whole |
|||||||||
|
Abdominal pain, generalized |
2.4 (3) |
3.3 (4) |
5.0 (6) |
1.7 (3) |
2.4 (4) |
||||
|
Asthenia/fatigue |
16.3 (20) |
15.4 (19) |
16.0 (19) |
8.1 (14) |
5.3 (9) |
||||
|
Fever |
2.4 (3) |
1.6 (2) |
3.4 (4) |
6.4 (11) |
7.1 (12) |
||||
|
Flu syndrome |
4.9 (6) |
7.3 (9) |
5.0 (6) |
5.2 (9) |
2.4 (4) |
||||
|
Headache |
14.6 (18) |
12.2 (15) |
16.8 (20) |
12.8 (22) |
11.2 (19) |
||||
|
Localized pain |
4.9 (6) |
5.7 (7) |
5.0 (6) |
2.9 (5) |
1.8 (3) |
||||
|
Digestive |
|||||||||
|
Diarrhea |
8.1 (10) |
2.4 (3) |
4.2 (5) |
8.1 (14) |
5.9 (10) |
||||
|
Nausea |
17.1 (21) |
20.3 (25) |
16.8 (20) |
9.3 (16) |
14.7 (25) |
||||
|
Vomiting |
8.9 (11) |
4.9 (6) |
2.5 (3) |
4.1 (7) |
6.5 (11) |
||||
|
Nervous |
|||||||||
|
Anxiety |
1.6 (2) |
2.4 (3) |
6.7 (8) |
4.1 (7) |
3.5 (6) |
||||
|
Depressive symptoms |
6.5 (8) |
4.9 (6) |
12.6 (15) |
3.5 (6) |
5.9 (10) |
||||
|
Insomnia |
4.9 (6) |
4.9 (6) |
5.0 (6) |
2.9 (5) |
1.2 (2) |
||||
|
Respiratory |
|||||||||
|
Bronchitis |
4.1 (5) |
6.5 (8) |
6.7 (8) |
3.5 (6) |
3.5 (6) |
||||
|
Cough |
9.8 (12) |
4.1 (5) |
5.0 (6) |
5.2 (9) |
3.5 (6) |
||||
|
Pharyngitis |
6.5 (8) |
1.6 (2) |
5.0 (6) |
4.1 (7) |
3.5 (6) |
||||
|
Sinusitis |
8.9 (11) |
7.3 (9) |
5.0 (6) |
2.3 (4) |
1.2 (2) |
||||
|
Upper respiratory infection |
11.4 (14) |
6.5 (8) |
7.6 (9) |
8.7 (15) |
4.7 (8) |
||||
|
Skin |
|||||||||
|
Rashes |
3.3 (4) |
19.5 (24) |
13.4 (16) |
7.6 (13) |
18.8 (32) |
||||
Other Adverse Events in Phase II/III Studies:
Other adverse events that occurred in patients receiving RESCRIPTOR (in combination treatment) in all Phase II and III studies, considered possibly related to treatment, and of at least ACTG Grade 2 in intensity are listed below by body system.
Body as a Whole: Abdominal cramps, abdominal distention, abdominal pain (localized), abscess, allergic reaction, chills, edema (generalized or localized), epidermal cyst, fever, infection, infection viral, lip edema, malaise, Mycobacterium tuberculosis infection, neck rigidity, sebaceous cyst, and redistribution/accumulation of body fat (see PRECAUTIONS: Fat Redistribution).
Cardiovascular System: Abnormal cardiac rate and rhythm, cardiac insufficiency, cardiomyopathy, hypertension, migraine, pallor, peripheral vascular disorder, and postural hypotension.
Digestive System: Anorexia, bloody stool, colitis, constipation, decreased appetite, diarrhea (Clostridium difficile), diverticulitis, dry mouth, dyspepsia, dysphagia, enteritis at all levels, eructation, fecal incontinence, flatulence, gagging, gastroenteritis, gastroesophageal reflux, gastrointestinal bleeding, gastrointestinal disorder, gingivitis, gum hemorrhage, hepatomegaly, increased appetite, increased saliva, increased thirst, jaundice, mouth or tongue inflammation or ulcers, nonspecific hepatitis, oral/enteric moniliasis, pancreatitis, rectal disorder, sialadenitis, tooth abscess, and toothache.
Hemic and Lymphatic System: Adenopathy, bruising, eosinophilia, granulocytosis, leukopenia, pancytopenia, purpura, spleen disorder, thrombocytopenia, and prolonged prothrombin time.
Metabolic and Nutritional Disorders: Alcohol intolerance, amylase increased, bilirubinemia, hyperglycemia, hyperkalemia, hypertriglyceridemia, hyperuricemia, hypocalcemia, hyponatremia, hypophosphatemia, increased AST (SGOT), increased gamma glutamyl transpeptidase, increased lipase, increased serum alkaline phosphatase, increased serum creatinine, and weight increase or decrease.
Musculoskeletal System: Arthralgia or arthritis of single and multiple joints, bone disorder, bone pain, myalgia, tendon disorder, tenosynovitis, tetany, and vertigo.
Nervous System: Abnormal coordination, agitation, amnesia, change in dreams, cognitive impairment, confusion, decreased libido, disorientation, dizziness, emotional lability, euphoria, hallucination, hyperesthesia, hyperreflexia, hypertonia, hypesthesia, impaired concentration, manic symptoms, muscle cramp, nervousness, neuropathy, nystagmus, paralysis, paranoid symptoms, restlessness, sleep cycle disorder, somnolence, tingling, tremor, vertigo, and weakness.
Respiratory System: Chest congestion, dyspnea, epistaxis, hiccups, laryngismus, pneumonia, and rhinitis.
Skin and Appendages: Angioedema, dermal leukocytoclastic vasculitis, dermatitis, desquamation, diaphoresis, discolored skin, dry skin, erythema, erythema multiforme, folliculitis, fungal dermatitis, hair loss, herpes zoster or simplex, nail disorder, petechiae, non-application site pruritus, seborrhea, skin hypertrophy, skin disorder, skin nodule, Stevens-Johnson syndrome, urticaria, vesiculobullous rash, and wart.
Special Senses: Blepharitis, blurred vision, conjunctivitis, diplopia, dry eyes, ear pain, parosmia, otitis media, photophobia, taste perversion, and tinnitus.
Urogenital System: Amenorrhea, breast enlargement, calculi of the kidney, chromaturia, epididymitis, hematuria, hemospermia, impaired urination, impotence, kidney pain, metrorrhagia, nocturia, polyuria, proteinuria, testicular pain, urinary tract infection, and vaginal moniliasis.
Postmarketing Experience:
Adverse event terms reported from postmarketing surveillance that were not reported in the Phase II and III trials are presented below.
Digestive System: Hepatic failure.
Hemic and Lymphatic System: Hemolytic anemia.
Musculoskeletal System: Rhabdomyolysis.
Urogenital System: Acute kidney failure.
Laboratory Abnormalities:
Marked laboratory abnormalities observed in at least 2% of patients during Studies 21 Part II and 13C are summarized in Table 10. Marked laboratory abnormalities are defined as any Grade 3 or 4 abnormality found in patients at any time during study.
| N/A = not applicable because no predose values were obtained for patients. | |||||
|
Adverse Events/Toxicity Limits |
Study 21 Part II |
Study 13C |
|||
|
Zidovudine + Lamivudine (n = 123) |
400 mg t.i.d. RESCRIPTOR + Zidovudine (n = 123) |
400 mg t.i.d. RESCRIPTOR + Zidovudine + Lamivudine (n = 119) |
Zidovudine + Didanosine, Zalcitabine, or Lamivudine (n = 172) |
400 mg t.i.d. RESCRIPTOR + Zidovudine + Didanosine, Zalcitabine, or Lamivudine (n = 170) |
|
|
% pts. |
% pts. |
% pts. |
% pts. |
% pts. |
|
|
Hematology |
|||||
|
Hemoglobin <7 mg/dL |
4.1 |
2.5 |
0.9 |
1.7 |
2.9 |
|
Neutrophils <750/mm3 |
5.7 |
4.9 |
3.4 |
10.4 |
7.6 |
|
Prothrombin time (PT) >1.5 × ULN |
0 |
0 |
1.7 |
2.9 |
2.4 |
|
Activated partial thromboplastin (APTT) >2.33 × ULN |
0 |
0.8 |
0 |
5.8 |
2.4 |
|
Chemistry |
|||||
|
Alananine aminotransferase (ALT/SGPT) >5 × ULN |
2.5 |
4.1 |
5.1 |
3.5 |
4.1 |
|
Amylase >2 × ULN |
0.8 |
2.5 |
2.6 |
3.5 |
2.9 |
|
Aspartate aminotransferase (AST/SGOT) >5 × ULN |
1.6 |
2.5 |
3.4 |
3.5 |
2.3 |
|
Bilirubin >2.5 × ULN |
0.8 |
2.5 |
1.7 |
1.2 |
0 |
|
Gamma glutamyl transferase (GGT) >5 × ULN |
N/A |
N/A |
N/A |
4.1 |
1.8 |
|
Glucose (hypo-/hyperglycemia) <40 mg/dL >250 mg/dL |
4.1 |
0.8 |
1.7 |
1.2 |
0 |
OVERDOSAGE
Human experience of acute overdose with RESCRIPTOR is limited.
Management of Overdosage:
Treatment of overdosage with RESCRIPTOR should consist of general supportive measures, including monitoring of vital signs and observation of the patient’s clinical status. There is no specific antidote for overdosage with RESCRIPTOR. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage. Since delavirdine is extensively metabolized by the liver and is highly protein-bound, dialysis is unlikely to result in significant removal of the drug.
DOSAGE AND ADMINISTRATION
The recommended dosage for RESCRIPTOR tablets is 400 mg (two 200-mg tablets) 3 times daily. RESCRIPTOR should be used in combination with other antiretroviral therapy. The complete prescribing information for other antiretroviral agents should be consulted for information on dosage and administration.
RESCRIPTOR tablets should be taken as intact tablets and may be administered with or without food (see CLINICAL PHARMACOLOGY: Pharmacokinetics: Absorption and Bioavailability). Patients with achlorhydria should take RESCRIPTOR with an acidic beverage (e.g., orange or cranberry juice). However, the effect of an acidic beverage on the absorption of delavirdine in patients with achlorhydria has not been investigated.
Patients taking both RESCRIPTOR and antacids should be advised to take them at least 1 hour apart.
HOW SUPPLIED
RESCRIPTOR tablets are available as follows:
200-mg: white, capsule-shaped tablets marked with “RES200”
Bottles of 180 tablets - NDC 49702-225-17.
Store at controlled room temperature 20° to 25°C (68° to 77°F) [see USP]. Keep container tightly closed. Protect from high humidity.
ANIMAL TOXICOLOGY
Toxicities among various organs and organ systems in rats, mice, rabbits, dogs, and monkeys were observed following the administration of delavirdine. Necrotizing vasculitis was the most significant toxicity that occurred in dogs when mean nadir serum concentrations of delavirdine were at least 7-fold higher than the expected human exposure to RESCRIPTOR (Cmin 15 microM) at the recommended dose. Vasculitis in dogs was not reversible during a 2.5-month recovery period; however, partial resolution of the vascular lesion characterized by reduced inflammation, diminished necrosis, and intimal thickening occurred during this period. Other major target organs included the gastrointestinal tract, endocrine organs, liver, kidneys, bone marrow, lymphoid tissue, lung, and reproductive organs.
RESCRIPTOR is a trademark owned by or licensed to the ViiV Healthcare group of companies.
The other brands listed are trademarks owned by or licensed to their respective owners and are not trademarks owned by or licensed to the ViiV Healthcare group of companies. The makers of these brands are not affiliated with and do not endorse the ViiV Healthcare group of companies or its products.
Manufactured for
ViiV Healthcare
Research Triangle Park, NC 27709
by
Pfizer Pharmaceuticals LLC
Vega Baja, Puerto Rico 00693
©2019 ViiV Healthcare group of companies or its licensor.
August 2019
RES:5PI
PATIENT INFORMATION
(delavirdine mesylate) tablets
Generic name: delavirdine mesylate (de-LAH-vur-deen MESS-ihl-ate)
ALERT: Find out about medicines that should NOT be taken with RESCRIPTOR. Please also read the section "MEDICINES YOU SHOULD NOT TAKE WITH RESCRIPTOR."
Read this information carefully before taking RESCRIPTOR. Also, read this leaflet each time you renew the prescription, just in case anything has changed. This is a summary and not a replacement for a careful discussion with your healthcare provider (doctor, nurse, pharmacist). You and your healthcare provider should discuss RESCRIPTOR when you start taking this medication and at regular checkups. You should remain under a doctor's care when taking RESCRIPTOR and should not change or stop treatment without first talking with your healthcare provider.
What is RESCRIPTOR and how does it work?
RESCRIPTOR is a medicine used in combination with other anti-HIV medicines to treat people with HIV-1 infection. Infection with HIV-1 leads to the destruction of infection-fighting immune system cells (called CD4+ cells or T cells), which are important to the immune system. After a large number of CD4+ cells have been destroyed, the infected person develops acquired immune deficiency syndrome (AIDS).
RESCRIPTOR helps to block HIV-1 reverse transcriptase, a chemical the virus uses to make more copies of itself. The main goals of anti-HIV medicines like RESCRIPTOR are to decrease the amount of virus in your blood (called viral load) and to increase the number of CD4+ cells as much as possible for as long as possible.
RESCRIPTOR, when taken with other anti-HIV medicines, lowers the HIV-1 viral load in patients. Patients who took RESCRIPTOR as part of combination therapy for HIV-1 also had increases in their CD4+ cell count.
General information about RESCRIPTOR
RESCRIPTOR does not cure HIV-1 or AIDS and you may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. You should remain under the care of a doctor when using RESCRIPTOR.
Avoid doing things that can spread HIV-1 infection.
- •
- Do not share needles or other injection equipment.
- •
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- •
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
How should I take RESCRIPTOR?
- •
- You should stay under a healthcare provider's care when taking RESCRIPTOR. Do not change your treatment or stop treatment without first talking with your healthcare provider.
- •
- You must take RESCRIPTOR every day exactly as your healthcare provider prescribes it. Follow the directions from your healthcare provider, exactly as written on the label.
- •
- The usual dose of RESCRIPTOR is two 200‑mg tablets 3 times a day, in combination with other anti-HIV-1 medicines. Your total daily dose of RESCRIPTOR remains the same.
- •
- You can take RESCRIPTOR with or without food.
- •
- The 200‑mg tablets must be swallowed whole. They cannot be dissolved in water.
- •
- Many people find it easier to take their RESCRIPTOR with breakfast, lunch, and dinner, since food does not interfere with RESCRIPTOR. It is a good idea to get into the habit of taking RESCRIPTOR on a regular schedule to make it easier to remember. Figure out things that happen every day at pill-taking time and take your tablets then. By taking your medicine along with activities you do every day, such as getting up in the morning, brushing your teeth, eating lunch, coming home from work in the evening, or watching a favorite TV show, you will find it easier to remember to take every dose.
- •
- When your supply of RESCRIPTOR starts to run low, get more from your healthcare provider or pharmacy. This is very important because the amount of virus in your blood may increase if the medicine is stopped for even a short time. The virus may develop resistance to RESCRIPTOR and become harder to treat.
- •
- Only take medicine that has been prescribed specifically for you. Do not give RESCRIPTOR to others or take medicine prescribed for someone else.
What should I do if I miss a dose of RESCRIPTOR?
If you forget to take a dose of RESCRIPTOR, take it as soon as possible. However, if you skip the dose entirely, do not double the next dose. If you forget a lot of doses, talk to your healthcare provider about how you should continue taking your medicine.
Who should not take RESCRIPTOR?
Together with your healthcare provider, you need to decide whether RESCRIPTOR is right for you.
- •
- Do not take RESCRIPTOR if you are taking certain medicines. These could cause serious side effects that could cause death. Before you take RESCRIPTOR, you must tell your healthcare provider about all the medicines you are taking or are planning to take. These include other prescription and nonprescription medicines and herbal supplements.
- For more information about medicines you should not take with RESCRIPTOR, please read the section titled "MEDICINES YOU SHOULD NOT TAKE WITH RESCRIPTOR."
- •
- Do not take RESCRIPTOR if you have an allergy to RESCRIPTOR. Also tell your healthcare provider if you have any known allergies to other medicines, foods, preservatives, or dyes.
- •
- Tell your healthcare provider if you are pregnant or plan to become pregnant. The effects of RESCRIPTOR on pregnant women or their unborn babies are not known.
- •
- If you are breastfeeding, do not breastfeed. We do not know if RESCRIPTOR can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk. Talk with your healthcare provider about the best way to feed your baby.
- •
- Talk with your healthcare provider if you have liver or kidney disease. RESCRIPTOR has not been studied in people with liver or kidney disease.
- •
- Certain medical problems may affect the use of RESCRIPTOR. Be sure to tell your healthcare provider of any other medical problems you may have.
Can I take RESCRIPTOR with other medicines?
RESCRIPTOR may interact with other medicines, including those you take without a prescription. You must tell your healthcare provider about all medicines you are taking or planning to take before you take RESCRIPTOR. It is a good idea to keep a complete list of all the medicines that you take, including nonprescription medicines, herbal remedies and supplements, and street drugs. Update this list when medicines are added or stopped. Give copies of this list to all of your healthcare providers every time you visit or fill a prescription.
MEDICINES YOU SHOULD NOT TAKE WITH RESCRIPTOR
Do not take the following medicines with RESCRIPTOR because they can cause serious problems or death if taken with RESCRIPTOR:
- •
- VERSED (midazolam) injection and syrup (for sedation)
- •
- HALCION (triazolam) tablets (for sleep problems)
- •
- XANAX (alprazolam) tablets (for anxiety)
- •
- D.H.E. 45 injection, ERGOMAR, MIGRANAL, WIGRAINE, and CAFERGOT (for migraine headaches)
- •
- METHERGINE (for bleeding after childbirth)
- •
- ORAP (pimozide) tablets (for seizures)
- •
- PROPULSID (cisapride) tablets and suspension (for heartburn)
- •
- HISMANAL (astemizole) tablets (for allergies)
- •
- SELDANE (terfenadine) tablets (for allergies)
Do not take the following medicines when you take RESCRIPTOR. They may reduce the levels of RESCRIPTOR in the blood and make it less effective. Talk with your healthcare provider if you are currently taking these medicines because other medicines may have to be given to take their place:
- •
- Rifampin (also known as RIMACTANE, RIFADIN, RIFATER, RIFAMATE) (to treat tuberculosis)
- •
- Phenobarbital (for seizures)
- •
- DILANTIN (phenytoin) (for seizures)
- •
- TEGRETOL (carbamazepine) (for seizures)
Do not take RESCRIPTOR with St. John's wort (Hypericum perforatum), an herbal product sold as a dietary supplement, or products containing St. John's wort. Talk with your healthcare provider if you are taking or planning to take St. John's wort. Taking St. John's wort may decrease levels of RESCRIPTOR and lead to increased viral load and possible resistance to RESCRIPTOR or cross-resistance to other anti-HIV medicines.
Do not take RESCRIPTOR with cholesterol-lowering medicines MEVACOR (lovastatin) or ZOCOR (simvastatin) because of possible serious reactions. There is also an increased risk of drug interactions between RESCRIPTOR and LIPITOR (atorvastatin), BAYCOL (cerivastatin), or LESCOL (fluvastatin); talk to your healthcare provider before you take any cholesterol-reducing medicines with RESCRIPTOR.
Medicines that require dosage adjustments:
It is possible that your healthcare provider may need to increase or decrease the dose of other medicines when you are taking RESCRIPTOR. Remember to tell your healthcare provider about all of the medicines you are taking or planning to take.
Before you take VIAGRA (sildenafil) with RESCRIPTOR, talk to your healthcare provider about problems these 2 medicines can cause when taken together. You may get increased side effects of VIAGRA, such as low blood pressure, vision changes, and penis erection lasting more than 4 hours. If an erection lasts longer than 4 hours, get medical help right away to avoid permanent damage to your penis. Your healthcare provider can explain these symptoms to you.
- •
- If you are taking both VIDEX (didanosine, ddI) and RESCRIPTOR: Take VIDEX (buffered tablets) 1 hour before or 1 hour after you take RESCRIPTOR. Taking them together causes lower amounts of RESCRIPTOR in the blood, making both medicines less effective.
- •
- Protease inhibitors: A number of healthy volunteers and HIV-1-infected patients were studied while taking RESCRIPTOR with one of these protease inhibitors: CRIXIVAN (indinavir), INVIRASE and FORTOVASE (saquinavir), NORVIR (ritonavir), or VIRACEPT (nelfinavir). RESCRIPTOR was shown to increase the amount of these protease inhibitors in the blood. RESCRIPTOR is expected to increase the amount of AGENERASE (amprenavir) and KALETRA (lopinavir + ritonavir) in the blood. As a result, your healthcare provider may choose to lower the dose of one of these medicines or monitor certain lab tests if these protease inhibitors are taken in combination with RESCRIPTOR.
- •
- Antacids should be taken at least 1 hour before or 1 hour after you take RESCRIPTOR because they can slow the absorption of RESCRIPTOR.
Based on your history of taking other anti-HIV medicine, your healthcare provider will direct you on how to take RESCRIPTOR and other anti-HIV medicines. These drugs should be taken in a certain order or at specific times. This will depend on how many times a day each medicine should be taken. It will also depend on whether the medicines should be taken with or without food.
What are the possible side effects of RESCRIPTOR?
- •
- This list of side effects is not complete. If you have questions about side effects, ask your doctor, nurse, or pharmacist. You should report any new or continuing symptoms to your healthcare provider right away. Your healthcare provider may be able to help you manage these side effects.
- •
- The most important common side effect seen in people taking RESCRIPTOR has been a skin rash. The rash occurs mainly on the upper body and upper arms, and sometimes on the neck and face. The rash appears as a red area on the skin with slight bumps, and it can be itchy. The rash tends to occur early, usually within 1 to 3 weeks after you start taking RESCRIPTOR, and it usually lasts less than 2 weeks. Watch your rash carefully and talk to your healthcare provider about how to treat it. If the rash is going to be serious or severe (with fever, blistering, sores in the mouth, redness or swelling of the eyes, or muscle and joint aches), you and your healthcare provider will usually realize it during the first 3 days of the rash. If you have symptoms of a severe rash, you should stop taking RESCRIPTOR and speak with your healthcare provider as soon possible. Be prepared to explain where the rash is, your temperature, and whether or not you have other symptoms.
- •
- Other side effects include headache, nausea, diarrhea, and tiredness. Of these, nausea was the most common.
- •
- Changes in body fat have been seen in some patients taking antiretroviral therapy. These changes may include increased amount of fat in the upper back and neck ("buffalo hump"), breast, and around the trunk. Loss of fat from the legs, arms and face may also happen. The cause and long-term health effects of these conditions are not known at this time.
- •
- Before you start using any medicine, talk with your healthcare provider about what to expect and discuss ways to reduce the side effects you may have.
How do I store RESCRIPTOR?
- •
- Keep RESCRIPTOR and all other medicines out of the reach of children. Keep the bottle closed and store at room temperature (between 68°F and 77°F) away from sources of moisture such as a sink or other damp place. Heat and moisture may reduce the effectiveness of RESCRIPTOR.
- •
- Do not keep medicine that is out of date or that you no longer need. Be sure that if you throw any medicine away, it is out of the reach of children.
General advice about prescription medicines:
Discuss all questions about your health with your healthcare provider. If you have questions about RESCRIPTOR or any other medicines you are taking, ask your healthcare provider. You can also call 1-877-844-8872 toll free.
AGENERASE and RESCRIPTOR are trademarks owned by or licensed to the ViiV Healthcare group of companies.
The other brands listed are trademarks owned by or licensed to their respective owners and are not trademarks owned by or licensed to the ViiV Healthcare group of companies. The makers of these brands are not affiliated with and do not endorse the ViiV Healthcare group of companies or its products.
Manufactured for
ViiV Healthcare
Research Triangle Park, NC 27709
by
Pfizer Pharmaceuticals LLC
Vega Baja, Puerto Rico 00693
©2019 ViiV Healthcare group of companies or its licensor.
August 2019
RES:5PIL
Principal Display Panel
NDC 49702-209-24
RESCRIPTOR®
delavirdine mesylate tablets
100 mg
360 Tablets
Rx only
ALERT: Find out about medicines that should NOT be taken with Rescriptor.
Note to Pharmacist: Do not cover ALERT box with pharmacy label.
Store at controlled room temperature 20° to 25°C (68° to 77°F) [see USP].
Keep container tightly closed. Protect from high humidity.
DOSAGE AND USE:
See prescribing information for dosage information.
Each tablet contains 100mg delavirdine mesylate.
Rev. 10/10
Manufactured for
ViiV Healthcare
Research Triangle Park, NC 27709
by
Pfizer Pharmaceuticals LLC
Vega Baja, Puerto Rico 00693
11342301
Principal Display Panel
NDC 49702-225-17
RESCRIPTOR®
delavirdine mesylate tablets
200 mg
180 Tablets
Rx only
ALERT: Find out about medicines that should NOT be taken with Rescriptor.
Note to Pharmacist: Do not cover ALERT box with pharmacy label.
Store at controlled room temperature 20° to 25°C (68° to 77°F) [see USP].
Keep container tightly closed.
Protect from high humidity.
DOSAGE AND USE:
See prescribing information for dosage information.
Each tablet contains 200mg delavirdine mesylate.
Rev. 3/12
Manufactured for
ViiV Healthcare
Research Triangle Park, NC 27709
by
Pfizer Pharmaceuticals LLC
Vega Baja, Puerto Rico 00693
11369501
| RESCRIPTOR
delavirdine mesylate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
 |
||||||||||||||||||
|
||||||||||||||||||
| RESCRIPTOR
delavirdine mesylate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - ViiV Healthcare Company (027295585) |