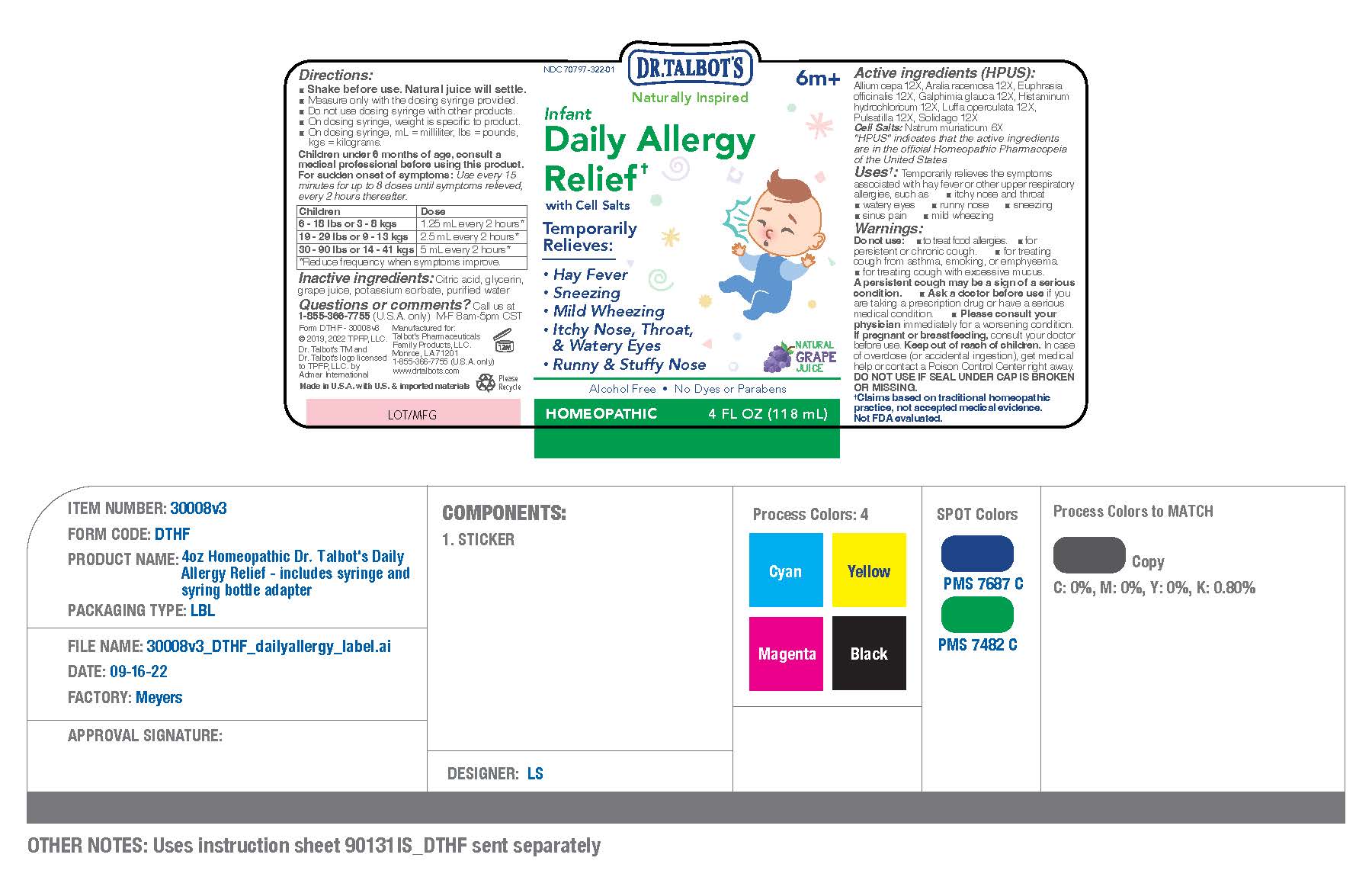
Label: DR TALBOTS INFANT DAILY ALLERGY RELIEF- allium cepa 12x,aralia racemosa 12x,euphrasia officinalis 12x,galphimia glauca 12x,histaminum hydrochloricum 12x,luffa operculata 12x,pulsatilla 12x,solidago 12x,natrum muriaticum 6x liquid
- NDC Code(s): 70797-322-01
- Packager: Talbot's Pharmaceuticals Family Products,LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- INACTIVE INGREDIENTS
- PURPOSE
- PREGNANCY
-
DIRECTIONS
 For sudden onset of symptoms:
For sudden onset of symptoms:
Use every 15 minutes for up to
8 doses until symptoms relieved,
then every 2 hours thereafter.
Children
6 - 18 lbs
or 3 - 8 kgs
1.25 mL every 2 hours,
reduce frequency when
symptoms improveChildren
19 - 29 lbs
or 9 - 13 kg2.5 mL every 2 hours,
reduce frequency when
symptoms improve
Children
30 - 90 lbs
or 14 - 41 kgs5 mL every 2 hours,
reduce frequency when
symptoms improve - QUESTIONS
- ASK A DOCTOR
- Warnings
- Uses
- Keep Out of Reach of Children
- PRINCIPAL DISPLAY
-
INGREDIENTS AND APPEARANCE
DR TALBOTS INFANT DAILY ALLERGY RELIEF
allium cepa 12x,aralia racemosa 12x,euphrasia officinalis 12x,galphimia glauca 12x,histaminum hydrochloricum 12x,luffa operculata 12x,pulsatilla 12x,solidago 12x,natrum muriaticum 6x liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70797-322 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARALIA RACEMOSA ROOT (UNII: T90W4582DU) (ARALIA RACEMOSA ROOT - UNII:T90W4582DU) ARALIA RACEMOSA ROOT 12 [hp_X] in 118 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 12 [hp_X] in 118 mL LUFFA OPERCULATA FRUIT (UNII: C4MO6809HU) (LUFFA OPERCULATA FRUIT - UNII:C4MO6809HU) LUFFA OPERCULATA FRUIT 12 [hp_X] in 118 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 12 [hp_X] in 118 mL ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 12 [hp_X] in 118 mL HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 12 [hp_X] in 118 mL SOLIDAGO VIRGAUREA FLOWERING TOP (UNII: 5405K23S50) (SOLIDAGO VIRGAUREA FLOWERING TOP - UNII:5405K23S50) SOLIDAGO VIRGAUREA FLOWERING TOP 12 [hp_X] in 118 mL GALPHIMIA GLAUCA FLOWERING TOP (UNII: 93PH5Q8M7E) (GALPHIMIA GLAUCA FLOWERING TOP - UNII:93PH5Q8M7E) GALPHIMIA GLAUCA FLOWERING TOP 12 [hp_X] in 118 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 6 [hp_X] in 118 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) CONCORD GRAPE JUICE (UNII: F7039Q79LP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70797-322-01 1 in 1 CARTON 09/12/2022 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/12/2022 Labeler - Talbot's Pharmaceuticals Family Products,LLC. (078855555)