MEDIHALER-ERGOTAMINE- ergotamine tartrate aerosol
3M Company
----------
DESCRIPTION
MEDIHALER ERGOTAMINE (ergotamine tartrate) is an oral aerosol device which contains a fine particle suspension of 9.0 mg ergotamine tartrate per ml in an insert, nontoxic aerosol vehicle. The inert ingredients are dichlorodifluoromethane, dichlorotetrafluoroethane, sorbitan trioleate and trichloromonofluoromethane. Each depression of the valve delivers a measured dose of 0.36 mg to the patient.
INDICATIONS
As therapy to abort vascular headache, e.g., migraine, migraine variants, or so-called “histaminic cephalalgia.”
CONTRAINDICATIONS
Ergotamine tartrate should not be used in the presence of coronary artery disease, peripheral vascular disease, hypertension, impaired renal or hepatic function, infectious states or malnutrition.
Ergotamine tartrate is contraindicated in patients with a history of hypersensitivity reactions.
WARNINGS
Patients who are being treated with MEDIHALER ERGOTAMINE should be informed adequately of the symptoms of ergotism. Close medical supervision by the physician is recommended so that he may react appropriately should signs of ergotism develop. Six inhalations per day, if continued daily, entail the risk of vasospastic complications. Avoid prolonged administration or in excess of the recommended dosage because of the danger of ergotism and gangrene.
ADVERSE REACTIONS
Ergotamine tartrate may cause nausea and vomiting. Patients with headaches may become nauseated and drug induced distress may be difficult to evaluate.
Ergotamine in large doses raises arterial pressure, produces coronary vasoconstriction and slows the heart both by direct action and its effect on the vagus. Under this condition, ergotamine also has oxytocic and spasmolytic properties. The above conditions are seen as a consequence of overdosage and may be manifested at the dosage recommended for control of headaches.
Vasoconstrictive complications, at times of a serious nature, may occur. These include pulselessness, weakness, muscle pains and paresthesias of the extremities and precordial distress and pain. Although these effects occur most commonly with long term therapy at relatively high doses, they have also been reported with short term or normal doses. Other adverse effects include transient tachycardia or bradycardia, nausea, vomiting, localized edema and itching.
DOSAGE AND ADMINISTRATION
Adults: A single oral inhalation at the first sign of a headache or prodrome. Repeat this procedure in 5 minutes if relief is not obtained. Space and additional inhalations at no less than 5-minute intervals. No more than 6 inhalations should be administered in any 24-hour period, and no more than 15 in a one week period.
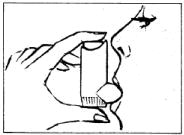
DIRECTIONS FOR USE:
Before each use, remove dust cap and shake MEDIHALER ERGOTAMINE.
- Breathe out fully and place mouthpiece well into the mouth, aimed at the back of the throat.
- As you begin to breathe in deeply, press the vial firmly down into the adapter with the index finger. This releases one dose.
- Release pressure on the vial and remove unit from mouth. Hold the breath as long as possible, then breathe out slowly.
HOW SUPPLIED
Metal vial (2.5 ml) and adapter. Each depression of the valve delivers 0.36 mg ergotamine tartrate.
(NDC 0089-0762-21)
CONTENTS UNDER PRESSURE
Do not puncture or incinerate container. Do not expose to heat or store at temperatures above 120°F. KEEP OUT OF THE REACH OF CHILDREN.
3M Riker
3M Health Care
Northridge, CA 91324
ERG-16
JUNE 1989
INSTRUCTIONS FOR USE OF
Medihaler Ergotamine®
(ergotamine tartrate)
FOR ORAL INHALATION THERAPY ONLY
SHAKE WELL before each use.
To get the medication deep into lungs, follow these five simple steps. (can be completed within 5 seconds):
- Remove dust cap and inspect mouthpiece for dirt and foreign objects.
- Empty the lungs as completely as possible by exhaling, and insert open end of plastic mouthpiece into mouth.
- After beginning to inhale deeply, press the top of the metal vial downward, as you would a push-button; this releases one measured dose of the medication. Complete taking the deep breath, which draws medication into your lungs.
- Remove mouthpiece and hold breath for as long as possible. This distributes the medication in the lungs
- Exhale slowly, keeping the lips nearly closed.
Start with one inhalation – then wait five minutes; if not relieved, use MEDIHALER ERGOTAMINE again. Any additional inhalations should be spaced at intervals of no less than five minutes. Take no more than six inhalations in any 24-hour period, and no more than 15 in a one-week period. MEDIHALER ERGOTAMINE was designed to deliver a full dose for most people in one or two inhalations. Do not use MEDIHALER ERGOTAMINE indiscriminately; use only the number of inhalations actually required for relief.
The oral adapter (plastic mouthpiece) should be kept clean. Simply remove metal vial and wash oral adapter with soap and hot water and rinse thoroughly. Dry and replace the vial and the dust cap.
WARNING: CONTENTS UNDER PRESSURE.
Do not puncture or incinerate container. Do not expose to heat or store at temperatures above 120°F. Keep out of the reach of children.
Medihaler Ergotamine®
(ergotamine tartrate)
For speed of relief and convenience the MEDIHALER ERGOTAMINE oral inhaler comes ready-to-use. It consists of two parts:
- A metal vial, containing the medication, with a single dose valve, which fits into
- The oral adapter (plastic mouthpiece) with dust cap. The vial can be used only with MEDIHALER ERGOTAMINE oral adapter.
ADVANTAGES:
- Provides relief with seconds
- Assures a uniform, measured dose with each depression of the vial
- Economical; all the medication is utilized; no loss or waste
3M Riker
3M Health Care
Northridge, CA 91324
JUNE 1989
| MEDIHALER-ERGOTAMINE
ergotamine tartrate aerosol |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - 3M Company (006173082) |