CYPROHEPTADINE HYDROCHLORIDE- cyproheptadine hydrochloride tablet
Apnar Pharma
----------
Cyproheptadine Hydrochloride Tablets, USP
DESCRIPTION
Cyproheptadine HCl USP is an antihistaminic and antiserotonergic agent.
Cyproheptadine hydrochloride USP is a white to slightly yellowish crystalline solid, with a molecular weight of 350.89, which is soluble in water, freely soluble in methanol, sparingly soluble in ethanol, soluble in chloroform, and practically insoluble in ether. It is the sesquihydrate of 4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine hydrochloride. The molecular formula of the anhydrous salt is C
21H
21N•HCl and the structural formula of the anhydrous salt is:
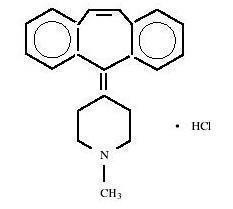
C 21H 21N• HCl M.W. 350.89
Cyproheptadine hydrochloride USP is available for oral administration in 4 mg tablets. Inactive ingredients include: lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
CLINICAL PHARMACOLOGY
Cyproheptadine is a serotonin and histamine antagonist with anticholinergic and sedative effects. Antiserotonin and antihistamine drugs appear to compete with serotonin and histamine, respectively, for receptor sites.
Pharmacokinetics and Metabolism
After a single 4 mg oral dose of 14C-labelled cyproheptadine HCl in normal subjects, given as tablets, 2 to 20% of the radioactivity was excreted in the stools. Only about 34% of the stool radioactivity was unchanged drug, corresponding to less than 5.7% of the dose. At least 40% of the administered radioactivity was excreted in the urine. No detectable amounts of unchanged drug were present in the urine of patients on chronic 12 to 20 mg daily doses. The principle metabolite found in human urine has been identified as a quaternary ammonium glucuronide conjugate of cyproheptadine. Elimination is diminished in renal insufficiency.
| CYPROHEPTADINE HYDROCHLORIDE
cyproheptadine hydrochloride tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Apnar Pharma (079568229) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| InvaTech Pharma Solutions LLC | 078602180 | manufacture(24689-816) , analysis(24689-816) | |

