SODIUM CHLORIDE- sodium chloride injection
Baxter Healthcare Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
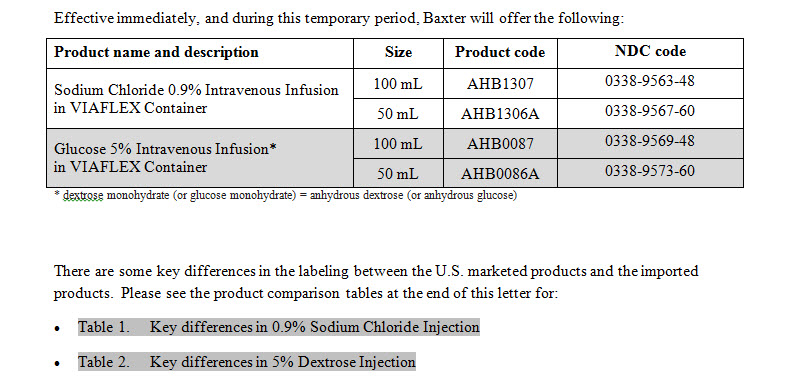
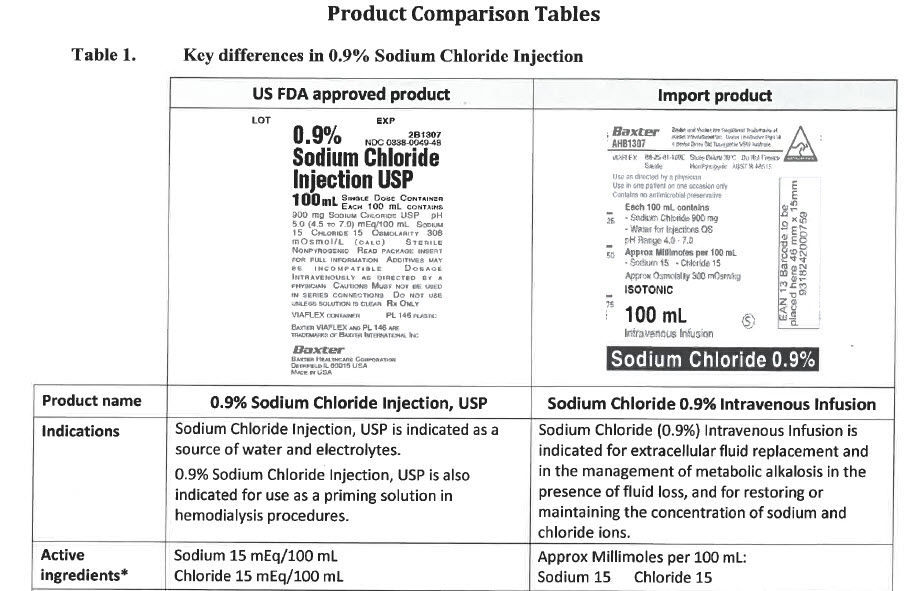
Sodium Chloride 0.9% Intravenous Infusion
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Baxter Logo
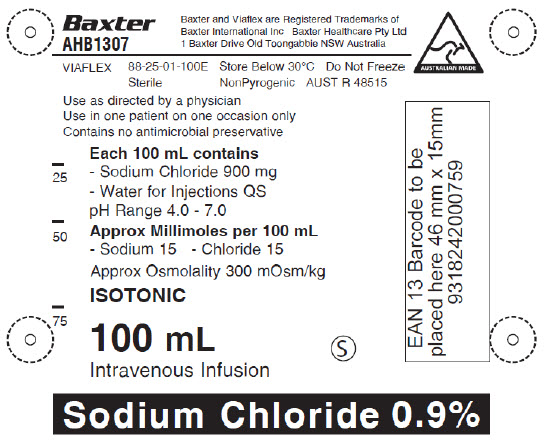
AHB1307
Baxter and Viaflex are Registered Trademarks of
Baxter International Inc Baxter Healthcare Pty Ltd
1 Baxter Drive Old Toongabbie NSW Australia
VIAFLEX 88-25-01-100E
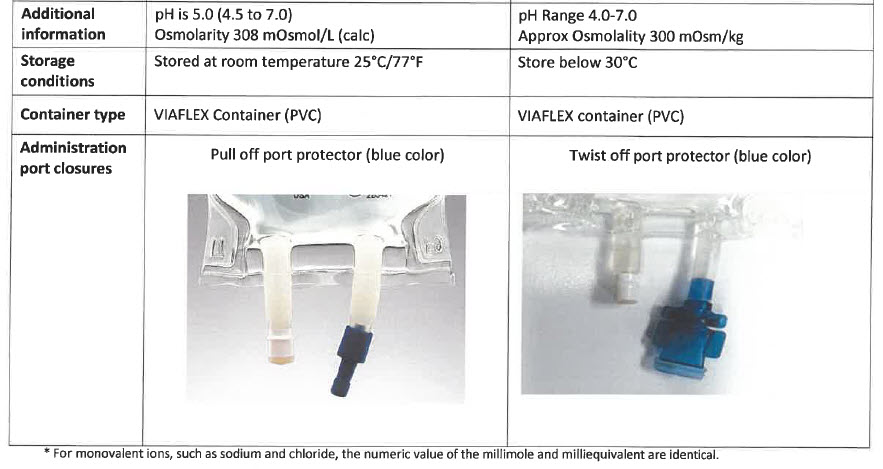
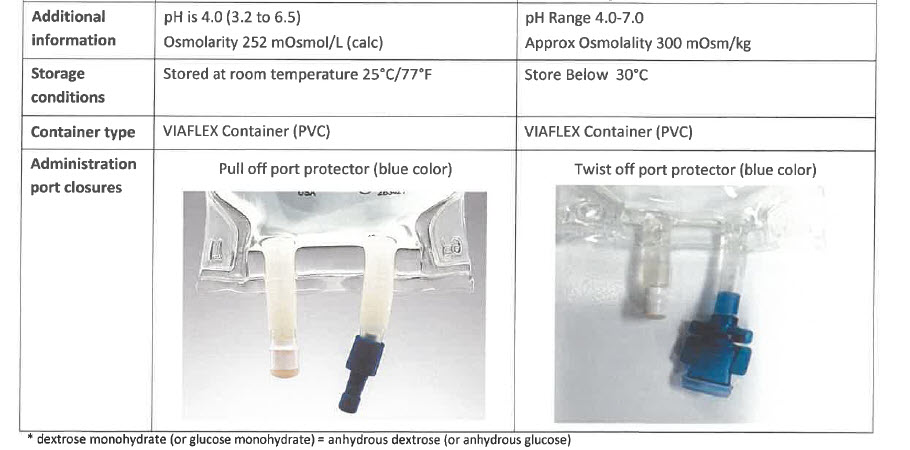
Store Below 30°C
Do Not Freeze
Sterile
NonPyrogenic
AUST R 48515
AUSTRALIAN MADE LOGO
Use as directed by a physician
Use in one patient on one occasion only
Contains no antimicrobial preservative
25
50
75
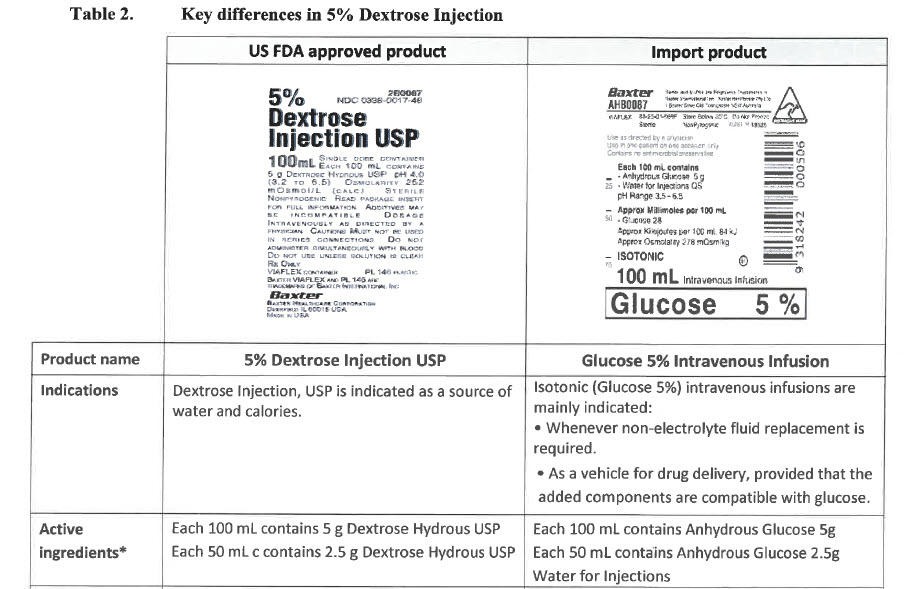
Each 100 mL contains
- Sodium Chloride 900 mg
- Water for Injections QS
pH Range 4.0 – 7.0
Approx Millimoles per 100 mL
- Sodium 15 - Chloride 15
Approx Osmolality 300 mOsm/kg
ISOTONIC
100 mL
Intravenous Infusion
Sodium Chloride 0.9%
EAN 13 Barcode to be
placed here 46 mm x 15mm
9318242000759
Baxter Logo
AHB1306A
Baxter and Viaflex are Registered Trademarks of
Baxter International Inc Baxter Healthcare Pty Ltd
1 Baxter Drive Old Toongabbie NSW Australia
VIAFLEX 88-25-01-101D
Store Below 30°C
Do Not Freeze
Sterile
NonPyrogenic
AUST R 19477
AUSTRALIAN MADE LOGO
Use as directed by a physician
Use in one patient on one occasion only
Contains no antimicrobial preservative
25
Each 50 mL contains
- Sodium Chloride 450 mg
- Water for Injections QS
pH Range 4.0 – 7.0
Approx Millimoles per 50 mL
- Sodium 7.5 - Chloride 7.5
Approx Osmolality 300 mOsm/kg
ISOTONIC
50 mL
Intravenous Infusion
Sodium Chloride 0.9%
EAN 13 Barcode to be
placed here 46 mm x 15mm
0085412311395
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| SODIUM CHLORIDE
sodium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare Pty Ltd | 750455891 | ANALYSIS(0338-9563, 0338-9567) , LABEL(0338-9563, 0338-9567) , MANUFACTURE(0338-9563, 0338-9567) , PACK(0338-9563, 0338-9567) , STERILIZE(0338-9563, 0338-9567) | |