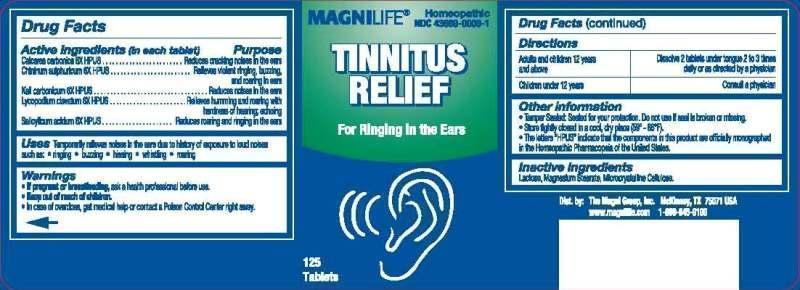
TINNITUS RELIEF- calcarea carbonica, chininum sulphuricum, kali carbonicum, lycopodium clavatum, salicylicum acidum tablet
The Magni Company
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
DRUG FACTS:
Calcarea Carbonica 6X, Chininum Sulphuricum 6X, Kali Carbonicum 6X, Lycopodium Clavatum 6X, Salicylicum Acidum 6X
INDICATIONS:
Temporarily relieves noises in the ears due to history of exposure to loud noises such as,
ringining, buzzing, hissing, whistling, roaring.
WARNINGS:
If pregnant or breastfeeding, ask a health professional before use. Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults and children 12 years and above, Dissolve 2 tablets under tongue 2 to 3 times daily or as directed by a physician.
Children under 12 years, consult a physician.
INACTIVE INGREDIENTS:
Lactose (Fast Flo Lactose USP 316), Magnesium Stearate, Microcrystalline Cellulose
KEEP OUT OF REACH OF CHILDREN:
KEEP OUT OF REACH OF CHILDREN: In case of overdose, get medical help or contact a Poison Control Center right away.
| TINNITUS RELIEF
calcarea carbonica, chininum sulphuricum, kali carbonicum, lycopodium clavatum, salicylicum acidum tablet |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - The Magni Company (113501902) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(43689-0009) , api manufacture(43689-0009) , label(43689-0009) , pack(43689-0009) | |