Label: OXYGEN gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 57439-003-01, 57439-003-02, 57439-003-03, 57439-003-04, view more57439-003-05, 57439-003-06, 57439-003-07, 57439-003-08, 57439-003-09, 57439-003-10, 57439-003-11, 57439-003-12, 57439-003-13, 57439-003-14, 57439-003-15 - Packager: Rotech Healthcare Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
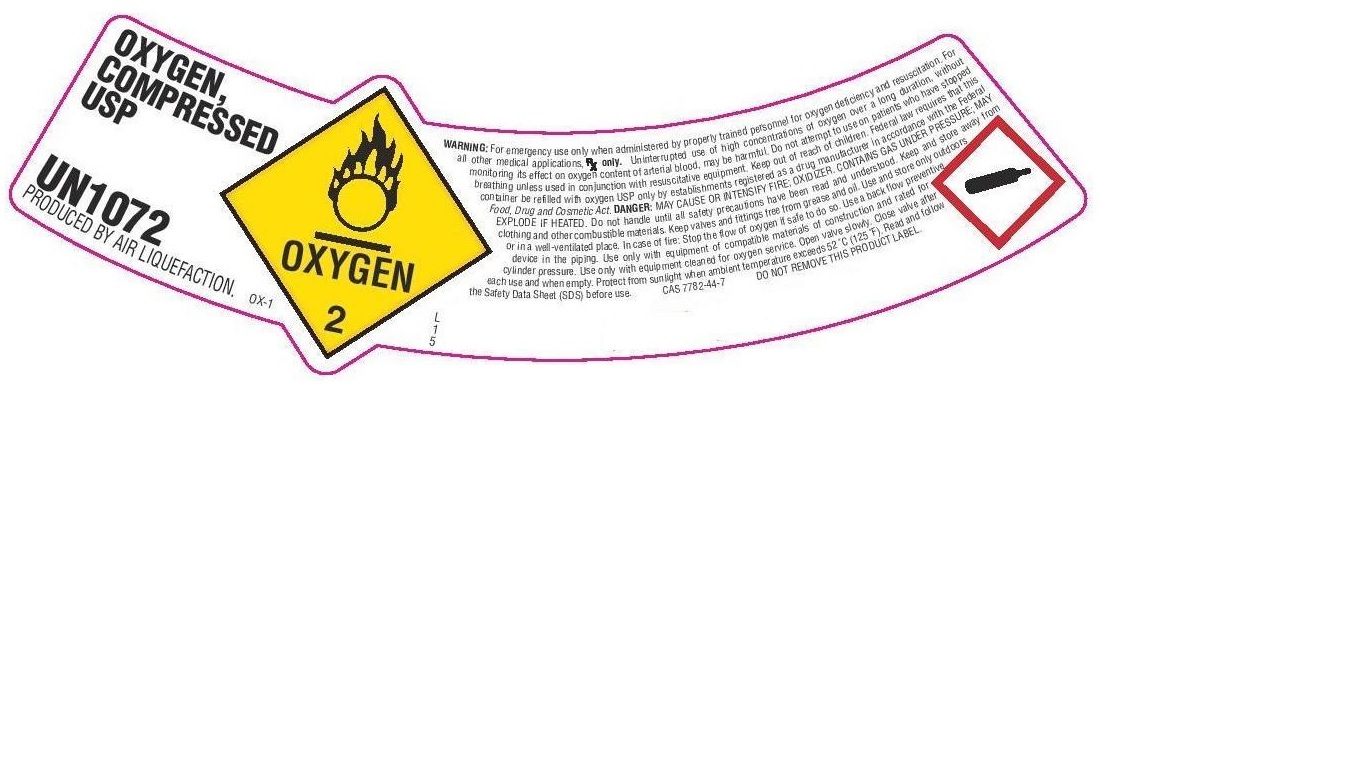
PRINCIPAL DISPLAY PANEL
OXYGEN, COMPRESSED USP
UN1072
PRODUCED BY AIR LIQUEFACTION
WARNING: For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx only. Uninterrupted use of high concentrations of oxygen over a long duration, without monitoring its effect on oxygen content of arterial blood, may be harmful. Do not attempt to use on patients who have stopped breathing unless used in conjunction with resuscitative equipment. Keep out of reach of children. Federal law requires that this container be refilled with oxygen USP only by establishments registered as a drug manufacturer in accordance with the Federal Food, Drug and Cosmetic Act. DANGER: MAY CAUSE OR INTENSIFY FIRE; OXIDIZER. CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED. Do not handle until all safety precautions have been read and understood. Keep and store away from clothing and other combustible materials. Keep valves and fittings free from grease and oil. Use and store only outdoors or in a well-ventilated place. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. Open valve slowly. Close valve after each use and when empty. Protect from sunlight when ambient temperature exceeds 52 C (125 F). Read and follow the Safety Data Sheet (SDS) before use. CAS 7782-44-7 DO NOT REMOVE THIS PRODUCT LABEL.
-
PRINCIPAL DISPLAY PANEL
OXYGEN, REFRIGERATED LIQUID USP
UN1073
PRODUCED BY AIR LIQUEFACTION
WARNING: For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx only. Uninterrupted use of high concentrations of oxygen over a long duration, without monitoring its effect on oxygen content of arterial blood, may be harmful. Do not attempt to use on patients who have stopped breathing unless used in conjunction with resuscitative equipment. Keep out of reach of children. Federal law requires that this container be refilled with oxygen USP only be establishments registered as a drug manufacturer in accordance with the Federal Food, Drug, and Cosmetic Act.
DANGER: MAY CAUSE OR INTENSIFY FIRE; OXIDIZER. CONTAINS REFRIGERATED GAS; MAY CAUSE CRYOGENIC BURNS OR INJURY. COMBUSTIBLES IN CONTACT WITH LIQUID OXYGEN MAY EXPLODE ON IGNITION OR IMPACT. Do not handle until all safety precautions have been read and understood. Keep and store away from clothing and other combustible materials. Keep valves and fittings free from grease and oil. Use and store only outdoors or in well-ventilated place. Wear cold insulating gloves, face shield, and eye protection. In case of fire: Stop the flow of oxygen if safe to do so. Use a back flow preventive device in the piping. Use only with equipment of compatible materials of construction and rated for cylinder pressure. Use only with equipment cleaned for oxygen service. DO NOT change or force fit connections. Avoid spills. Do not walk on or roll equipment over spills. Close valve after each use and when empty. Always keep container in upright position. Read and follow the Safety Data Sheet (SDS) before use.
FIRST AID: IF ON SKIN: Thaw frosted parts with lukewarm water. Do not rub affected area. Get immediate medical advice/attention. CAS: 7782-44-7
DO NOT REMOVE THIS PRODUCT LABEL

-
INGREDIENTS AND APPEARANCE
OXYGEN
oxygen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:57439-003 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYGEN (UNII: S88TT14065) (OXYGEN - UNII:S88TT14065) OXYGEN 990 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57439-003-08 3455 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/2018 2 NDC:57439-003-09 7100 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/2018 3 NDC:57439-003-10 9430 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/2018 4 NDC:57439-003-01 113 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/2018 5 NDC:57439-003-02 170 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/2018 6 NDC:57439-003-03 255 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/2018 7 NDC:57439-003-04 425 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/2018 8 NDC:57439-003-05 680 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/2018 9 NDC:57439-003-06 1738 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/2018 10 NDC:57439-003-07 2549 L in 1 CYLINDER; Type 0: Not a Combination Product 01/01/2018 11 NDC:57439-003-11 20 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/2018 12 NDC:57439-003-12 30 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/2018 13 NDC:57439-003-13 32 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/2018 14 NDC:57439-003-14 37 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/2018 15 NDC:57439-003-15 45 L in 1 DEWAR; Type 0: Not a Combination Product 01/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205889 01/01/2018 Labeler - Rotech Healthcare Inc (144556222) Registrant - Rotech Healthcare Inc (144556222) Establishment Name Address ID/FEI Business Operations ROTECH 016953296 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations RHEMA MEDICAL 078670599 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations Oxygen Plus, Inc. dba Summit Respiratory 079569918 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations HEARTLAND HOME HEALTH CARE 079570162 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH 079570216 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations MAJOR MEDICAL 079607390 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH 079607401 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations COMMUNITY HOME OXYGEN 079607617 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH HOME MEDICAL CARE 079607622 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations FIRST CARE 079639101 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH OF ARLINGTON HEIGHTS 079713679 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH 079721579 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations MAJOR MEDICAL SUPPLY 079721904 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH 079738728 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations KELLEY'S HOME HEALTH SERVICES 079738768 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations G&G MEDICAL 085400138 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH 098136096 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations SUMMIT RESPIRATORY 135922784 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations CPO2 148713329 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH 159346050 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations HOME CARE MEDICAL SERVICES 169932436 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations Sentry Home Health 174392501 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTH MEDICAL 179387050 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH 611480690 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations CAMBRIA MEDICAL SUPPLY 620007021 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations HOME MEDICAL SYSTEMS 795469142 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations SENTRY HOME HEALTH 796502123 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations G&G MEDICAL 796502479 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTH MEDICAL 831552364 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations SUMMIT RESPIRATORY 831552372 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH 831562918 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations FAMILY HOME CARE 831748491 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations GREAT LAKES HOME MEDICAL 831831172 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTECH 833186922 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations MED-AIR TRANSFILL 841973667 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations SUMMIT RESPIRATORY 874476422 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations ROTH MEDICAL 948744180 manufacture(57439-003) Establishment Name Address ID/FEI Business Operations VITALCARE HEALTH SERVICES 956721187 manufacture(57439-003)