CVS MERTHIOLATE- benzalkonium chloride liquid
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
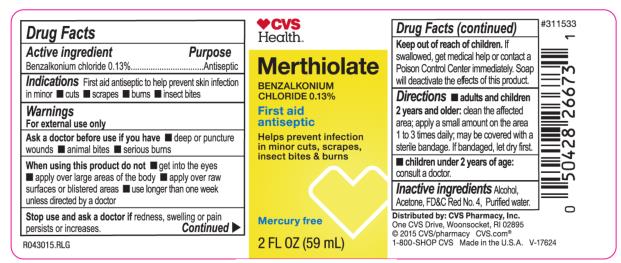
CVS Merthiolate
Indications
First aid antiseptic to help skin infection in minor: cuts, scrapes, burns, insect bites.
Warnings
For external use only.
| CVS MERTHIOLATE
benzalkonium chloride liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Humco Holding Group, inc. (825672884) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Humco Holding Group, Inc. | 825672884 | analysis(69842-749) , manufacture(69842-749) , pack(69842-749) , label(69842-749) | |
Revised: 12/2020
Document Id: b6d998cb-998e-2226-e053-2a95a90aec39
Set id: dd498ab1-8350-41aa-86fc-2db57005f3e2
Version: 3
Effective Time: 20201219
CVS Pharmacy