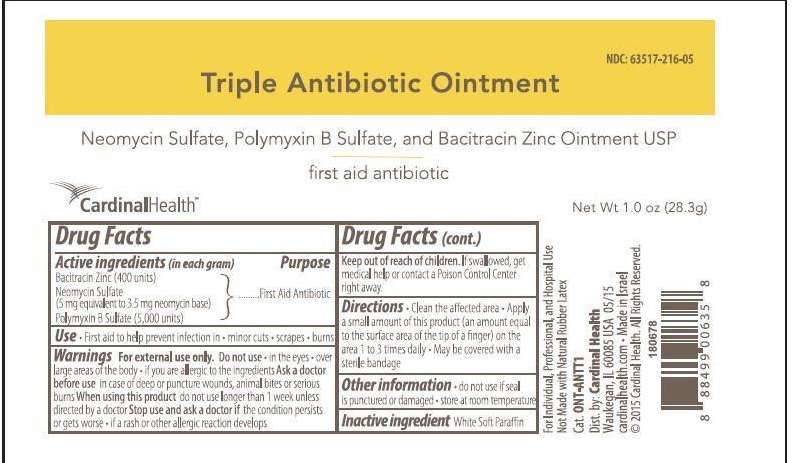
CARDINAL HEALTH FIRST AID ANTIBIOTIC- neomycin sulfate,polymyxin b sulfate,bacitracin zinc ointment
Cardinal Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Active ingredients (in each gram)
Bacitracin Zinc (400 units)
Neomycin Sulfate (5mg equivalent to 3.5 mg Neomycin base)
Polymyxin B Sulfate (5,000 units)
Warnings
For external use only.
| CARDINAL HEALTH
FIRST AID ANTIBIOTIC
neomycin sulfate,polymyxin b sulfate,bacitracin zinc ointment |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Cardinal Health (961027315) |
Revised: 1/2022
Document Id: d4bb08c7-7ece-4e62-e053-2a95a90a9cdd
Set id: dc83493b-0381-4ba8-a3f7-cb2dc41e5d87
Version: 3
Effective Time: 20220103
Cardinal Health