Label: PROGESTERONE capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 69387-101-01, 69387-102-01 - Packager: Banner Life Sciences LLC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 30, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
BOXED WARNING
WARNING: CARDIOVASCULAR DISORDERS, BREAST CANCER and PROBABLE DEMENTIA FOR ESTROGEN PLUS PROGESTIN THERAPY
Cardiovascular Disorders and Probable Dementia
Estrogens plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia. (See CLINICAL STUDIESand WARNINGS, Cardiovascular disorders and Probable dementia.)
The Women's Health Initiative (WHI) estrogen plus progestin substudy reported increased risks of deep vein thrombosis, pulmonary embolism, stroke and myocardial infarction in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg] combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo. (See CLINICAL STUDIES and WARNINGS, Cardiovascular disorders.)
The WHI Memory Study (WHIMS) estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women. (See CLINICAL STUDIES and WARNINGS, Probable dementia and PRECAUTIONS, Geriatric Use.)
Breast Cancer
The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer. (See CLINICAL STUDIES and WARNINGS, Malignant neoplasms, Breast Cancer.)
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and MPA, and other combinations and dosage forms of estrogens and progestins.
Progestins with estrogens should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
-
DESCRIPTION
Progesterone USP, capsules contain micronized progesterone for oral administration. Progesterone has a molecular weight of 314.47 and a molecular formula of C21H30O2. Progesterone (pregn-4-ene-3, 20-dione) is a white or creamy white, odorless, crystalline powder practically insoluble in water, soluble in alcohol, acetone and dioxane and sparingly soluble in vegetable oils, stable in air, melting between 126° and 131°C. The structural formula is:
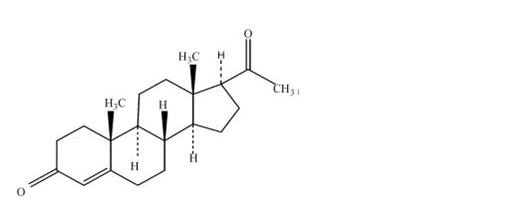
Progesterone is synthesized from a starting material from a plant source and is chemically identical to progesterone of human ovarian origin. Progesterone capsules are available in multiple strengths to afford dosage flexibility for optimum management. Progesterone capsules contain 100 mg or 200 mg micronized progesterone.
The inactive ingredients for progesterone, capsules 100 mg include: ferric oxide red NF, ferric oxide yellow NF, gelatin NF, glycerin USP, lecithin NF, peanut oil NF, titanium dioxide USP.
The inactive ingredients for progesterone, capsules 200 mg include: ferric oxide red NF, gelatin NF, glycerin USP, lecithin NF, peanut oil NF, titanium dioxide USP.
-
CLINICAL PHARMACOLOGY
Progesterone capsules are an oral dosage form of micronized progesterone which is chemically identical to progesterone of ovarian origin. The oral bioavailability of progesterone is increased through micronization.
Pharmacokinetics
A. Absorption
After oral administration of progesterone as a micronized soft-gelatin capsule formulation, maximum serum concentrations were attained within 3 hours. The absolute bioavailability of micronized progesterone is not known. Table 1 summarizes the mean pharmacokinetic parameters in postmenopausal women after five oral daily doses of progesterone capsules 100 mg as a micronized soft-gelatin capsule formulation.
TABLE 1. Pharmacokinetic Parameters of Progesterone Parameter
Progesterone capsules Daily Dose
100 mg
200 mg
300 mg
a Mean ± S.D. Cmax (ng/mL)
17.3 ± 21.9a
38.1 ± 37.8
60.6 ± 72.5
Tmax (hr)
1.5 ± 0.8
2.3 ± 1.4
1.7 ± 0.6
AUC (0-10) (ng × hr/mL)
43.3 ± 30.8
101.2 ± 66.0
175.7 ± 170.3
Serum progesterone concentrations appeared linear and dose proportional following multiple dose administration of progesterone capsules 100 mg over the dose range 100 mg/day to 300 mg/day in postmenopausal women. Although doses greater than 300 mg/day were not studied in females, serum concentrations from a study in male volunteers appeared linear and dose proportional between 100 mg/day and 400 mg/day. The pharmacokinetic parameters in male volunteers were generally consistent with those seen in postmenopausal women.
B. Distribution
Progesterone is approximately 96 percent to 99 percent bound to serum proteins, primarily to serum albumin (50 to 54 percent) and transcortin (43 to 48 percent).
C. Metabolism
Progesterone is metabolized primarily by the liver largely to pregnanediols and pregnanolones. Pregnanediols and pregnanolones are conjugated in the liver to glucuronide and sulfate metabolites. Progesterone metabolites which are excreted in the bile may be deconjugated and may be further metabolized in the gut via reduction, dehydroxylation, and epimerization.
D. Excretion
The glucuronide and sulfate conjugates of pregnanediol and pregnanolone are excreted in the bile and urine. Progesterone metabolites are eliminated mainly by the kidneys. Progesterone metabolites which are excreted in the bile may undergo enterohepatic recycling or may be excreted in the feces.
E. Special Populations
The pharmacokinetics of progesterone capsules have not been assessed in low body weight or obese patients.
Hepatic Insufficiency: The effects of hepatic impairment on progesterone capsules pharmacokinetics have not been studied.
Renal Insufficiency: The effects of renal impairment on progesterone capsules pharmacokinetics have not been studied.
F. Food–Drug Interaction
Concomitant food ingestion increased the bioavailability of progesterone capsules relative to a fasting state when administered to postmenopausal women at a dose of 200 mg.
G. Drug Interactions
The metabolism of progesterone by human liver microsomes was inhibited by ketoconazole (IC50 <0.1 μM). Ketoconazole is a known inhibitor of cytochrome P450 3A4, hence these data suggest that ketoconazole or other known inhibitors of this enzyme may increase the bioavailability of progesterone. The clinical relevance of the in vitro findings is unknown.
Coadministration of conjugated estrogens and progesterone capsules to 29 postmenopausal women over a 12-day period resulted in an increase in total estrone concentrations (Cmax 3.68 ng/mL to 4.93 ng/mL) and total equilin concentrations (Cmax 2.27 ng/mL to 3.22 ng/mL) and a decrease in circulating 17β estradiol concentrations (Cmax 0.037 ng/mL to 0.030 ng/mL). The half-life of the conjugated estrogens was similar with coadministration of progesterone capsules. Table 2 summarizes the pharmacokinetic parameters.
TABLE 2. Mean (± S.D.) Pharmacokinetic Parameters for Estradiol, Estrone, and Equilin Following coadministration of Conjugated Estrogens 0.625 mg and progesterone capsules 200 mg for 12 Days to Postmenopausal Women Conjugated Estrogens Conjugated Estrogens plus
progesterone capsulesa Total estrogens is the sum of conjugated and unconjugated estrogen. Drug
Cmax
(ng/mL)
Tmax (hr)
AUC(0-24h)
(ng × h/mL)
Cmax
(ng/mL)
Tmax
(hr)AUC(0-24h)
(ng × h/mL)
Estradiol
0.037 ±
0.04812.7 ±
9.10.676 ±
0.7370.030 ±
0.03217.32 ±
1.210.561 ± 0.572 Estrone
Totala
3.68 ±
1.5510.6 ±
6.861.3 ±
26.364.93 ± 2.07
7.5 ± 3.8
85.9 ± 41.2
Equilin
Totala
2.27 ± 0.95
6.0 ± 4.0
28.8 ± 13.0
3.22 ± 1.13
5.3 ± 2.6
38.1 ± 20.2
CLINICAL STUDIES
Effects on the endometrium
In a randomized, double-blind clinical trial, 358 postmenopausal women, each with an intact uterus, received treatment for up to 36 months. The treatment groups were: progesterone capsules at the dose of 200 mg/day for 12 days per 28-day cycle in combination with conjugated estrogens 0.625 mg/day (n=120); conjugated estrogens
0.625 mg/day only (n=119); or placebo (n=119). The subjects in all three treatment groups were primarily Caucasian women (87 percent or more of each group). The results for the incidence of endometrial hyperplasia in women receiving up to 3 years of treatment are shown in Table 3. A comparison of the progesterone capsules plus conjugated estrogens treatment group to the conjugated estrogens only group showed a significantly lower rate of hyperplasia (6 percent combination product versus 64 percent estrogen alone) in the progesterone capsules plus conjugated estrogens treatment group throughout 36 months of treatment.
TABLE 3. Incidence of Endometrial Hyperplasia in Women Receiving 3 Years of Treatment Endometrial Diagnosis Treatment Group Conjugated Estrogens 0.625 mg + Progesterone Capsules 200 mg (cyclical) Conjugated Estrogens 0.625 mg alone Placebo Number of patients % of patients Number of patients % of patients Number of patients % of patients n=117 n=115 n=116 a Most advanced result to least advanced result: Adenocarcinoma > atypical hyperplasia > complex hyperplasia >simple hyperplasia HYPERPLASIAa
7
6
74
64
3
3
Adenocarcinoma
0
0
0
0
1
1
Atypical hyperplasia
1
1
14
12
0
0
Complex hyperplasia
0
0
27
23
1
1
Simple hyperplasia
6
5
33
29
1
1
The times to diagnosis of endometrial hyperplasia over 36 months of treatment are shown in Figure 1. This figure illustrates graphically that the proportion of patients with hyperplasia was significantly greater for the conjugated estrogens group (64 percent) compared to the conjugated estrogens plus progesterone capsules group (6 percent).
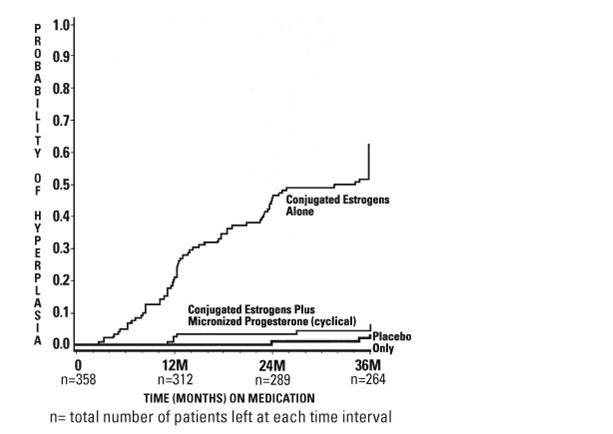
Figure 1. Time to Hyperplasia in Women Receiving up to 36 Months of Treatment
The discontinuation rates due to hyperplasia over the 36 months of treatment are as shown in Table 4. For any degree of hyperplasia, the discontinuation rate for patients who received conjugated estrogens plus progesterone capsules was similar to that of the placebo only group, while the discontinuation rate for patients who received conjugated estrogens alone was significantly higher. Women who permanently discontinued treatment due to hyperplasia were similar in demographics to the overall study population.
TABLE 4. Discontinuation Rate Due to Hyperplasia Over 36 Months of Treatment Most Advanced
Biopsy Result Through 36 Months
of TreatmentTreatment Group Conjugated Estrogens + Progesterone Capsules (cyclical) Conjugated Estrogens (alone) Placebo n=120 n=119 n=119 Number of
patients% of
patientsNumber of
patients% of
patientsNumber of
patients% of
patientsAdenocarcinoma
0
0
0
0
1
1
Atypical hyperplasia
1
1
10
8
0
0
Complex hyperplasia
0
0
21
18
1
1
Simple hyperplasia
1
1
13
11
0
0
Effects on secondary amenorrhea
In a single-center, randomized, double-blind clinical study that included premenopausal women with secondary amenorrhea for at least 90 days, administration of 10 days of progesterone capsules therapy resulted in 80 percent of women experiencing withdrawal bleeding within 7 days of the last dose of progesterone capsules, 300 mg/day (n=20), compared to 10 percent of women experiencing withdrawal bleeding in the placebo group (n=21).
In a multicenter, parallel-group, open label, postmarketing dosing study that included premenopausal women with secondary amenorrhea for at least 90 days, administration of 10 days of PROMETRIUM Capsules during two 28-day treatment cycles, 300 mg per day (n=107) or 400 mg per day (n=99), resulted in 73.8 percent and 76.8 percent of women, respectively, experiencing withdrawal bleeding.
The rate of secretory transformation was evaluated in a multicenter, randomized, double-blind clinical study in estrogen-primed postmenopausal women. Progesterone capsules administered orally for 10 days at 400 mg/day (n=22) induced complete secretory changes in the endometrium in 45 percent of women compared to 0 percent in the placebo group (n=23).
A second multicenter, parallel-group, open label postmarketing dosing study in premenopausal women with secondary amenorrhea for at least 90 days also evaluated the rate of secretory transformation. All subjects received daily oral conjugated estrogens over 3 consecutive 28-day treatment cycles and PROMETRIUM Capsules, 300 mg per day (n=107) or 400 mg per day (n=99) for 10 days of each treatment cycle. The rate of complete secretory transformation was 21.5 percent and 28.3 percent, respectively.
Women's Health Initiative Studies
The Women’s Health Initiative (WHI) enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of either the use of daily oral conjugated estrogens (CE) [0.625 mg] alone or in combination with medroxyprogesterone acetate (MPA) [2.5 mg] compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) (nonfatal myocardial infarction [MI], silent MI and CHD death), with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, pulmonary embolism (PE), endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. The study did not evaluate the effects of CE or CE plus MPA on menopausal symptoms.
WHI Estrogen Plus Progestin Substudy
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” The absolute excess risk of events in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women-years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the estrogen plus progestin substudy, which included 16,608 women (average age of 63 years, range 50 to 79; 83.9 percent White, 6.8 percent Black, 5.4 percent Hispanic, 3.9 percent Other) are presented in Table 5. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
TABLE 5. Relative and Absolute Risk Seen in the Estrogen Plus Progestin Substudy of WHI at an Average of 5.6 Yearsb Eventc Relative Risk CE/MPA versus Placebo (95% nCIb)
Placebo
n = 8,102
CE/MPA
n = 8,506
Absolute Risk per 10,000 Women-Years
a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi.
b Results are based on centrally adjudicated data.
c Nominal confidence intervals unadjusted for multiple looks and multiple comparisons.
d Not included in Global Index.
e Includes metastatic and non-metastatic breast cancer with the exception of in situ breast cancer.
f All deaths, except from breast or colorectal cancer, definite/probable CHD, PE or cerebrovascular disease.
g A subset of the events was combined in a “global index,”defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death due to other causes.CHD events
Non-fatal MIb
CHD death
1.23 (0.99-1.53)
1.28 (1.00-1.63)
1.10 (0.70-1.75)
34
25
8
41
31
8
All stroke
1.31 (1.03-1.88)
25
33
Ischemic stroke
1.44 (1.09-1.90)
18
26
Deep vein thrombosis d
1.95 (1.43-2.67)
13
26
Pulmonary embolism
2.13 (1.45-3.11)
8
18
Invasive breast cancer e
1.24 (1.01-1.54)
33
41
Colorectal cancer
0.61 (0.42-0.87)
16
10
Endometrial cancer d
0.82 (0.48-1.36)
7
6
Cervical cancer d
1.44 (0.47-4.42)
1
2
Hip fracture
0.67 (0.47-0.96)
16
11
Vertebral fractures d
0.68 (0.48-0.96)
17
12
Lower arm/wrist fractures d
0.71 (0.59-0.85)
62
44
Total fractures d
0.76 (0.69-0.83)
199
152
Overall mortality c, e
1.00 (0.83-1.19)
52
52
Global Index g
1.13 (1.02-1.25)
165
184
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified for age showed in women 50 to 59 years of age a non-significant trend toward reducing risk of overall mortality [hazard ratio (HR) 0.69 (95 percent CI, 0.44-1.07)].
Women's Health Initiative Memory Study
The estrogen plus progestin Women’s Health Initiative Memory Study (WHIMS), an ancillary study of WHI, enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years of age; and 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared with placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21– to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 per 10,000 women-years. Probable dementia as defined in this study included Alzheimer's disease (AD), vascular dementia (VaD) and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See WARNINGS, Probable dementia and PRECAUTIONS, Geriatric Use.)
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Progesterone capsules should not be used in women with any of the following conditions:
- Progesterone capsules should not be used in patients with known hypersensitivity to itsingredients. Progesterone capsules contain peanut oil and should never be used by patients allergic to peanuts.
- Undiagnosed abnormal genital bleeding.
- Known, suspected, or history of breast cancer.
- Active deep vein thrombosis, pulmonary embolism or history of these conditions.
- Active arterial thromboembolic disease (for example, stroke and myocardial infarction), or a history of these conditions.
- Known liver dysfunction or disease.
- Known or suspected pregnancy.
-
WARNINGS
See BOXED WARNING.
1. Cardiovascular disorders
An increased risk of pulmonary embolism, deep vein thrombosis (DVT), stroke, and myocardial infarction has been reported with estrogen plus progestin therapy. Should any of these occur or be suspected, estrogen with progestin should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (for example, personal history or family history of venous thromboembolism [VTE], obesity, and systemic lupus erythematosus) should be managed appropriately.
a. Stroke
In the Women’s Health Initiative (WHI) estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). The increase in risk was demonstrated after the first year and persisted. (See CLINICAL STUDIES.) Should a stroke occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
b. Coronary Heart Disease
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of coronary heart disease (CHD) events (defined as nonfatal myocardial infarction [MI], silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women-years). An increase in relative risk was demonstrated in year 1 and a trend toward decreasing relative risk was reported in years 2 through 5. (See CLINICAL STUDIES.)
In postmenopausal women with documented heart disease (n = 2,763, average age 66.7 years), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study [HERS]), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established coronary heart disease. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred and twenty one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in HERS, HERS II, and overall.
c. Venous thromboembolism
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and pulmonary embolism [PE]) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (35 versus 17 per 10,000 women-years) and PE. Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women-years) were also demonstrated. The increase in VTE risk was observed during the first year and persisted. (See CLINICAL STUDIES.) Should a VTE occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
If feasible, estrogens with progestins should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
2. Malignant neoplasms
a. Breast cancer
The most important randomized clinical trial providing information about breast cancer in estrogen plus progestin users is the Women’s Health Initiative (WHI) substudy of daily CE (0.625 mg) plus MPA (2.5 mg). After a mean follow-up of 5.6 years, the estrogen plus progestin substudy reported an increased risk of breast cancer in women who took daily CE plus MPA. In this substudy, prior use of estrogen alone or estrogen plus progestin therapy was reported by 26 percent of the women. The relative risk of invasive breast cancer was 1.24 (95 percent nCI 1.01-1.54), and the absolute risk was 41 versus 33 cases per 10,000 women-years, for estrogen plus progestin compared with placebo.
Among women who reported prior use of hormone therapy, the relative risk of invasive breast cancer was 1.86, and the absolute risk was 46 versus 25 cases per 10,000 women-years, for estrogen plus progestin compared with placebo. Among women who reported no prior use of hormone therapy, the relative risk of invasive breast cancer was 1.09, and the absolute risk was 40 versus 36 cases per 10,000 women-years for estrogen plus progestin compared with placebo. In the same substudy, invasive breast cancers were larger, were more likely to be node positive, and were diagnosed at a more advanced stage in the CE (0.625 mg) plus MPA (2.5 mg) group compared with the placebo group. Metastatic disease was rare, with no apparent difference between the two groups. Other prognostic factors such as histologic subtype, grade and hormone receptor status did not differ between the groups. (See CLINICAL STUDIES.)
Consistent with the WHI clinical trials, observational studies have also reported an increased risk of breast cancer for estrogen plus progestin therapy, and a smaller increased risk for estrogen-alone therapy, after several years of use. The risk increased with duration of use, and appeared to return to baseline over about 5 years after stopping treatment (only the observational studies have substantial data on risk after stopping). Observational studies also suggest that the risk of breast cancer was greater, and became apparent earlier, with estrogen plus progestin therapy as compared to estrogen-alone therapy. However, these studies have not generally found significant variation in the risk of breast cancer among different estrogen plus progestin combinations, doses, or routes of administration.
The use of estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation. All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
b. Endometrial Cancer
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among unopposed estrogen users is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use, with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in all cases of undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy in postmenopausal women has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
c. Ovarian Cancer
The WHI estrogen plus progestin substudy reported a statistically non-significant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent nCI 0.77-3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years. In some epidemiologic studies, the use of estrogen-only products, in particular for 5 or more years, has been associated with an increased risk of ovarian cancer. However, the duration of exposure associated with increased risk is not consistent across all epidemiologic studies and some report no association.
3. Probable Dementia
In the estrogen plus progestin Women’s Health Initiative Memory Study (WHIMS), an ancillary study of WHI, a population of 4,532 postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
In the WHIMS estrogen plus progestin ancillary study, after an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for estrogen plus progestin versus placebo was 2.05 (95 percent CI 1.21– 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. It is unknown whether these findings apply to younger postmenopausal women. (See CLINICAL STUDIES and PRECAUTIONS, Geriatric Use.)
4. Vision abnormalities
Retinal vascular thrombosis has been reported in patients receiving estrogen. Discontinue estrogen plus progestin therapy pending examination if there is sudden partial or complete loss of vision, or if there is a sudden onset of proptosis, diplopia or migraine. If examination reveals papilledema or retinal vascular lesions, estrogen plus progestin therapy should be permanently discontinued.
-
PRECAUTIONS
A. General
1. Addition of a progestin when a woman has not had a hysterectomy
Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks that may be associated with the use of progestins with estrogens compared with estrogen-alone regimens. These include a possible increased risk of breast cancer.
2. Fluid Retention
Progesterone may cause some degree of fluid retention. Women with conditions that might be influenced by this factor, such as cardiac or renal dysfunction, warrant careful observation.
3. Dizziness and Drowsiness
Progesterone capsules may cause transient dizziness and drowsiness and should be used with caution when driving a motor vehicle or operating machinery. PROGESTERONE capsules should be taken as a single daily dose at bedtime.
B. Patient Information
General: This product contains peanut oil and should not be used if you are allergic to peanuts.
Physicians are advised to discuss the contents of the Patient Information leaflet with patients for whom they prescribe PROGESTERONE Capsules.
C. Drug-Laboratory Test Interactions
The following laboratory results may be altered by the use of estrogen plus progestin therapy:
- Increased sulfobromophthalein retention and other hepatic function tests.
- Coagulation tests: increase in prothrombin factors VII, VIII, IX and X.
- Pregnanediol determination.
- Thyroid function: increase in PBI, and butanol extractable protein bound iodine and decrease in T3 uptake values.
D. Carcinogenesis, Mutagenesis, Impairment of Fertility
Progesterone has not been tested for carcinogenicity in animals by the oral route of administration. When implanted into female mice, progesterone produced mammary carcinomas, ovarian granulosa cell tumors and endometrial stromal sarcomas. In dogs, long-term intramuscular injections produced nodular hyperplasia and benign and malignant mammary tumors. Subcutaneous or intramuscular injections of progesterone decreased the latency period and increased the incidence of mammary tumors in rats previously treated with a chemical carcinogen.
Progesterone did not show evidence of genotoxicity in in vitro studies for point mutations or for chromosomal damage. In vivo studies for chromosome damage have yielded positive results in mice at oral doses of 1000 mg/kg and 2000 mg/kg. Exogenously administered progesterone has been shown to inhibit ovulation in a number of species and it is expected that high doses given for an extended duration would impair fertility until the cessation of treatment.
E. Pregnancy
Progesterone capsules should not be used during pregnancy. (See CONTRAINDICATIONS.)
Pregnancy Category B: Reproductive studies have been performed in mice at doses up to 9 times the human oral dose, in rats at doses up to 44 times the human oral dose, in rabbits at a dose of 10 mcg/day delivered locally within the uterus by an implanted device, in guinea pigs at doses of approximately one-half the human oral dose and in rhesus monkeys at doses approximately the human dose, all based on body surface area, and have revealed little or no evidence of impaired fertility or harm to the fetus due to progesterone.
F. Nursing Women
Detectable amounts of progestin have been identified in the milk of nursing women receiving progestins. Caution should be exercised when progesterone capsules are administered to a nursing woman.
G. Pediatric Use
Progesterone capsules are not indicated in children. Clinical studies have not been conducted in the pediatric population.
H. Geriatric Use
There have not been sufficient numbers of geriatric women involved in clinical studies utilizing progesterone capsules to determine whether those over 65 years of age differ from younger subjects in their response to progesterone capsules.
The Women’s Health Initiative Study
In the Women’s Health Initiative (WHI) estrogen plus progestin substudy, (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age. (See WARNINGS, Cardiovascular Disorders and Malignant Neoplasms.)
The Women’s Health Initiative Memory Study
In the Women’s Health Initiative Memory Study (WHIMS) of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in the estrogen plus progestin ancillary study when compared to placebo. (See CLINICAL STUDIES and WARNINGS, Probable Dementia.)
-
ADVERSE REACTIONS
See BOXED WARNING, WARNINGS and PRECAUTIONS.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In a multicenter, randomized, double-blind, placebo-controlled clinical trial, the effects of progesterone capsules on the endometrium was studied in a total of 875 postmenopausal women. Table 6 lists adverse experiences greater than or equal to 2 percent of women who received cyclic progesterone capsules, 200 mg daily (12 days per calendar month cycle) with 0.625 mg conjugated estrogens or placebo.
Table 6. Adverse Experiences (≥2%) Reported in an 875 Patient Placebo-Controlled Trial in Postmenopausal Women Over a 3-Year Period [Percentage (%) of Patients Reporting] Progesterone capsules 200 mg with Congugated Estrogens
0.625 mg
Placebo
(n=178)
(n=174)
Headache
31
27
Breast Tenderness
27
6
Joint Pain
20
29
Depression
19
12
Dizziness
15
9
Abdominal Bloating
12
5
Hot Flashes
11
35
Urinary Problems
11
9
Abdominal Pain
10
10
Vaginal Discharge
10
3
Nausea / Vomiting
8
7
Worry
8
4
Chest Pain
7
5
Diarrhea
7
4
Night Sweats
7
17
Breast Pain
6
2
Swelling of Hands and Feet
6
9
Vaginal Dryness
6
10
Constipation
3
2
Breast Carcinoma
2
<1
Breast Excisional Biopsy
2
<1
Cholecystectomy
2
<1
Effects on Secondary Amenorrhea
In a multicenter, randomized, double-blind, placebo-controlled clinical trial, the effects of progesterone on secondary amenorrhea was studied in 49 estrogen-primed postmenopausal women. Table 7 lists adverse experiences greater than or equal to 5 percent of women who received progesterone or placebo.
Table 7. Adverse Experiences (≥5%) Reported in Patients Using 400 mg/day in a Placebo-Controlled Trial in Estrogen-Primed Postmenopausal Women Adverse Experience Progesterone Capsules 400 mg Placebo n=25 n=24
Percentage (%) of Patients
Fatigue
8
4
Headache
16
8
Dizziness
24
4
Abdominal Distention (Bloating)
8
8
Abdominal Pain (Cramping)
20
13
Diarrhea
8
4
Nausea
8
0
Back Pain
8
8
Musculoskeletal Pain
12
4
Irritability
8
4
Breast Pain
16
8
Infection Viral
12
0
Coughing
8
0
In a multicenter, parallel-group, open label postmarketing dosing study consisting of three consecutive 28-day treatment cycles, 220 premenopausal women with secondary amenorrhea were randomized to receive daily conjugated estrogens therapy (0.625 mg conjugated estrogens) and progesterone capsules, 300 mg per day (n=113) or Progesterone Capsules, 400 mg per day (n=107) for 10 days of each treatment cycle. Overall, the most frequently reported treatment-emergent adverse reactions, reported in greater than or equal to 5 percent of subjects, were nausea, fatigue, vaginal mycosis, nasopharyngitis, upper respiratory tract infection, headache, dizziness, breast tenderness, abdominal distension, acne, dysmenorrhea, mood swing, and urinary tract infection.
Postmarketing Experience
The following additional adverse reactions have been reported with progesterone capsules. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Genitourinary System: endometrial carcinoma, hypospadia, intra-uterine death, menorrhagia, menstrual disorder, metrorrhagia, ovarian cyst, spontaneous abortion.
Cardiovascular: circulatory collapse, congenital heart disease (including ventricular septal defect and patent ductus arteriosus), hypertension, hypotension, tachycardia.
Gastrointestinal: acute pancreatitis, cholestasis, cholestatic hepatitis, dysphagia, hepatic failure, hepatic necrosis, hepatitis, increased liver function tests (including alanine aminotransferase increased, aspartate aminotransferase increased, gammaglutamyl transferase increased), jaundice, swollen tongue.
Skin: alopecia, pruritus, urticaria.
Eyes: blurred vision, diplopia, visual disturbance.
Central Nervous System: aggression, convulsion, depersonalization, depressed consciousness, disorientation, dysarthria, loss of consciousness, paresthesia, sedation, stupor, syncope (with and without hypotension), transient ischemic attack, suicidal ideation.
During initial therapy, a few women have experienced a constellation of many or all of the following symptoms: extreme dizziness and/or drowsiness, blurred vision, slurred speech, difficulty walking, loss of consciousness, vertigo, confusion, disorientation, feeling drunk, and shortness of breath.
Miscellaneous: abnormal gait, anaphylactic reaction, arthralgia, blood glucose increased, choking, cleft lip, cleft palate, difficulty walking. dyspnea, face edema, feeling abnormal, feeling drunk, hypersensitivity, asthma, muscle cramp, throat tightness, tinnitus, vertigo, weight decreased, weight increased.
- OVERDOSAGE
-
DOSAGE & ADMINISTRATION
Prevention of Endometrial Hyperplasia
Progesterone capsules should be given as a single daily dose at bedtime, 200 mg orally for 12 days sequentially per 28-day cycle, to postmenopausal women with a uterus who are receiving daily conjugated estrogens tablets.
Treatment of Secondary Amenorrhea
Progesterone capsules may be given as a single daily dose of 400 mg at bedtime for 10 days.
Some women may experience difficulty swallowing progesterone capsules. For these women, progesterone capsules should be taken with a glass of water while in the standing position.
-
HOW SUPPLIED
Progesterone, capsules 100 mg are available as an oval orange, opaque, capsule imprinted with P-1 in black ink. NDC 69387-101-01 (Bottle of 100)
Progesterone, capsules 200 mg are available as an oval red, opaque, capsule imprinted with P-2 in black ink. NDC 69387-102-01 (Bottle of 100)
Store at 20-25°C (68-77°F). [See USP Controlled Room Temperature]
Protect from excessive moistureKeep out of reach of children.
Dispense in tight, light-resistant container as defined in USP/NF, accompanied by a
Patient Insert.
Manufactured by: Banner Pharmacaps Inc. High Point, NC 27265
November 2013
-
PATIENT INFORMATION
Progesterone, Capsules 100 mg
Progesterone, Capsules 200 mgRead this PATIENT INFORMATION before you start taking progesterone capsules and read what you get each time you refill your progesterone capsules prescription. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT PROGESTERONE CAPSULES (A Progesterone Hormone)?
- Progesterone with estrogens should not be used to prevent heart disease, heart attacks, strokes, or dementia.
- Using progestins with estrogens may increase your chance of getting heart attacks, strokes, breast cancer, and blood clots.
- Using progestins with estrogens may increase your chance of getting dementia, based on a study of women age 65 and older.
- You and your healthcare provider should talk regularly about whether you still need treatment with progesterone capsules.
THIS PRODUCT CONTAINS PEANUT OIL AND SHOULD NOT BE USED IF YOU ARE ALLERGIC TO PEANUTS.
What is progesterone capsules?
Progesterone capsules contain the female hormone called progesterone.
What is progesterone capsules used for?
Treatment of Menstrual Irregularities
Progesterone capsules are used for the treatment of secondary amenorrhea (absence of menstrual periods in women who have previously had a menstrual period) due to a decrease in progesterone. When you do not produce enough progesterone, menstrual irregularities can occur. If your healthcare provider has determined your body does not produce enough progesterone on its own, progesterone capsules may be prescribed to provide the progesterone you need.
Protection of the Endometrium (Lining of the Uterus)
Progesterone Capsules are used in combination with estrogen-containing medications in postmenopausal women with a uterus. Taking estrogens alone increases the chance of developing a condition called endometrial hyperplasia that may lead to cancer of the lining of the uterus (womb). The addition of a progestin is generally recommended for women with a uterus to reduce the chance of getting cancer of the uterus (womb).
Who should not take progesterone capsules?
Do not start taking progesterone capsules if you:
- Are allergic to peanuts.
- Have unusual vaginal bleeding.
- Currently have or have had certain cancers.
Estrogen plus progestin treatment may increase the chance of getting certain types of cancers, including cancer of the breast or uterus. If you have or have had cancer, talk with your healthcare provider about whether you should take progesterone capsules.
- Had a stroke or heart attack.
- Currently have or have had blood clots.
- Currently have or have had liver problems.
- Are allergic to any of the ingredients in progesterone capsules. See the list of ingredients at the end of this leaflet.
-
Think you may be pregnant.
Tell your healthcare provider:
- If you are breastfeeding. The hormones in progesterone capsules can pass into your breast milk.
- About all of your medical problems. Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood.
- About all the medicines you take. This includes prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may affect how progesterone capsules work. Progesterone capsules may also affect how your other medicines work.
How should I take progesterone capsules?
- Prevention of Endometrial Hyperplasia: A postmenopausal women with a uterus who is taking estrogens should take a single daily dose of 200 mg progesterone capsules at bedtime for 12 continuous days per 28-day cycle.
- Secondary Amenorrhea: Progesterone capsules may be given as a single daily dose of 400 mg at bedtime for 10 days.
- Progesterone capsules are to be taken at bedtime as some women become very drowsy and/or dizzy after taking progesterone capsules. In a few cases , symptoms may include blurred vision, difficulty speaking, difficulty walking, and feeling abnormal. If you experience these symptoms, discuss them with your healthcare provider right away.
If you experience difficulty in swallowing progesterone capsules, it is recommended that you take your daily dose at bedtime with a glass of water while in the standing position.
What are the possible side effects of progesterone capsules?
Side effects are grouped by how serious they are and how often they happen when you are treated:
Serious but less common side effects include:
- Risk to the Fetus: Cases of cleft palate, cleft lip, hypospadias, ventricular septal defect, patent ductus arteriosus, and other congenital heart defects.
- Abnormal Blood Clotting: stroke, heart attack, pulmonary embolus, visual loss or blindness.
Some of the warning signs of serious side effects include:
- Changes in vision or speech
- Sudden new severe headaches
- Severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
- Dizziness and faintness
- Vomiting
Call your healthcare provider right away if you get any of these warning signs, or any other unusual symptoms that concern you.
Less serious but common side effects include:
- Headaches
- Breast pain
- Irregular vaginal bleeding or spotting
- Stomach/abdominal cramps, bloating
- Nausea and vomiting
- Hair loss
- Fluid retention
- Vaginal yeast infection
These are not all the possible side effects of progesterone capsules. For more information, ask your healthcare provider or pharmacist for advice about side effects. You may report side effects at 1-866-231-1749 or to FDA at 1-800-FDA-1088.
What can I do to lower my chances of getting a serious side effect with progesterone capsules?
- Talk with your healthcare provider regularly about whether you should continue taking progesterone capsules.
- See your healthcare provider right away if you get unusual vaginal bleeding while taking progesterone capsules.
- Have a pelvic exam, breast exam, and mammogram (breast X-ray) every year unless your healthcare provider tells you something else. If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast exams more often.
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances for getting heart disease. Ask your healthcare provider for ways to lower your chances for getting heart disease.
General information about safe and effective use of progesterone capsules
- Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not take progesterone capsules for conditions for which it was not prescribed.
- Your healthcare provider has prescribed this drug for you and you alone. Do not give progesterone capsules to other people, even if they have the same symptoms you have. It may harm them.
- Progesterone capsules should be taken as a single daily dose at bedtime. Some women may experience extreme dizziness and/or drowsiness during initial therapy. In a few cases, symptoms may include blurred vision, difficulty speaking, difficulty walking, and feeling abnormal. If you experience these symptoms, discuss them with your healthcare provider right away.
- Use caution when driving a motor vehicle or operating machinery as dizziness or drowsiness may occur.
Keep progesterone capsules out of the reach of children.
This leaflet provides a summary of the most important information about progesterone capsules. If you would like more information, talk with your healthcare provider or pharmacist. You can ask for information about progesterone capsules that is written for health professionals. You can get more information by calling the toll free number 1-866-231-1749.
What are the ingredients in progesterone capsules?
Active ingredient: 100 mg or 200 mg micronized progesterone
The inactive ingredients for progesterone capsules 100 mg include: ferric oxide Red NF, ferric oxide yellow NF, gelatin NF, glycerin USP, lecithin NF, peanut oil NF, titanium dioxide USP.
The inactive ingredients for progesterone capsules 200 mg include: ferric oxide Red NF, gelatin NF, glycerin USP, lecithin NF, peanut oil NF, titanium dioxide USP.
Progesterone, Capsules 100 mg are available as an oval orange, opaque, capsule imprinted with P-1 in black ink. NDC 69387-101-01 (Bottle of 100 capsules)
Progesterone, Capsules 200 mg are available as an oval red, opaque, capsule imprinted with P-2 in black ink. NDC 69387-102-01 (Bottle of 100 capsules)
Store at 20-25°C (68-77°F).
Protect from excessive moisture
Manufactured for: Banner Life Sciences LLC.
September 2015
- PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label
- PRINCIPAL DISPLAY PANEL - 200 mg Capsule Bottle Label
-
INGREDIENTS AND APPEARANCE
PROGESTERONE
progesterone capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69387-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROGESTERONE (UNII: 4G7DS2Q64Y) (PROGESTERONE - UNII:4G7DS2Q64Y) PROGESTERONE 100 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PEANUT OIL (UNII: 5TL50QU0W4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE Score no score Shape OVAL Size 13mm Flavor Imprint Code P;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69387-101-01 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200900 10/01/2015 PROGESTERONE
progesterone capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69387-102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROGESTERONE (UNII: 4G7DS2Q64Y) (PROGESTERONE - UNII:4G7DS2Q64Y) PROGESTERONE 200 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PEANUT OIL (UNII: 5TL50QU0W4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color RED Score no score Shape OVAL Size 15mm Flavor Imprint Code P;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69387-102-01 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200900 10/01/2015 Labeler - Banner Life Sciences LLC. (079579273) Establishment Name Address ID/FEI Business Operations Banner Pharmacaps 945494508 MANUFACTURE(69387-101, 69387-102) , ANALYSIS(69387-101, 69387-102)




