Label: ANASTROZOLE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 42254-161-30 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 16729-035
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 29, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use anastrozole tablets safely and effectively. See full prescribing information for anastrozole tablets. Anastrozole tablet for oral use. Initial U.S. Approval: 1995
RECENT MAJOR CHANGES
----
INDICATIONS AND USAGE
Anastrozole tablet is an aromatase inhibitor indicated for:
- Adjuvant treatment of postmenopausal women with hormone receptor-positive early breast cancer (1.1)
- First-line treatment of postmenopausal women with hormone receptor-positive or hormone receptor unknown locally advanced or metastatic breast cancer (1.2)
- Treatment of advanced breast cancer in postmenopausal women with disease progression following tamoxifen therapy. Patients with ER-negative disease and patients who did not respond to previous tamoxifen therapy rarely responded to anastrozole tablets (1.3)
DOSAGE AND ADMINISTRATION
One 1 mg tablet taken once daily (2)
DOSAGE FORMS AND STRENGTHS
1 mg tablets (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- In women with pre-existing ischemic heart disease, an increased incidence of ischemic cardiovascular events occurred with anastrozole use compared to tamoxifen use. Consider risks and benefits. (5.1, 6.1)
- Decreases in bone mineral density may occur. Consider bone mineral density monitoring. (5.2, 6.1)
- Increases in total cholesterol may occur. Consider cholesterol monitoring. (5.3, 6.1)
ADVERSE REACTIONS
- In the early breast cancer (ATAC) study, the most common (occurring with an incidence of >10%) side effects occurring in women taking anastrozole included: hot flashes, asthenia, arthritis, pain, arthralgia, pharyngitis, hypertension, depression, nausea and vomiting, rash, osteoporosis, fractures, back pain, insomnia, headache, peripheral edema and lymphedema, regardless of causality. (6.1)
- In the advanced breast cancer studies, the most common (occurring with an incidence of >10%) side effects occurring in women taking anastrozole included: hot flashes, nausea, asthenia, pain, headache, back pain, bone pain, increased cough, dyspnea, pharyngitis and peripheral edema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Accord Healthcare Inc. at 1-866-941-7875 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pediatric patients: Efficacy has not been demonstrated for pubertal boys of adolescent age with gynecomastia or girls with McCune Albright Syndrome and progressive precocious puberty. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2010
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Adjuvant Treatment
1.2 First-Line Treatment
1.3 Second-Line Treatment
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Patients with Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Pregnancy and Premenopausal Women
4.2 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Ischemic Cardiovascular Events
5.2 Bone Effects
5.3 Cholesterol
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Tamoxifen
7.2 Estrogen
7.3 Warfarin
7.4 Cytochrome P450
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adjuvant Treatment of Breast Cancer in Postmenopausal Women
14.2 First Line Therapy in Postmenopausal Women with Advanced Breast Cancer
14.3 Second Line Therapy in Postmenopausal Women with Advanced Breast Cancer who had Disease Progression following Tamoxifen Therapy
16 HOW SUPPLIED/STORAGE AND HANDLING
Storage
17 PATIENT COUNSELING INFORMATION
17.1 Pregnancy
17.2 Allergic (Hypersensitivity) Reactions
17.3 Ischemic Cardiovascular Events
17.4 Bone Effects
17.5 Cholesterol
17.6 Tamoxifen
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Adjuvant Treatment
Anastrozole tablets are indicated for adjuvant treatment of postmenopausal women with hormone receptor-positive early breast cancer.
1.2 First-Line Treatment
Anastrozole tablets are indicated for the first-line treatment of postmenopausal women with hormone receptor-positive or hormone receptor unknown locally advanced or metastatic breast cancer.
1.3 Second-Line Treatment
Anastrozole tablets are indicated for the treatment of advanced breast cancer in postmenopausal women with disease progression following tamoxifen therapy. Patients with ER-negative disease and patients who did not respond to previous tamoxifen therapy rarely responded to anastrozole tablets.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The dose of anastrozole tablets is one 1 mg tablet taken once a day. For patients with advanced breast cancer, anastrozole tablets should be continued until tumor progression. Anastrozole tablets can be taken with or without food.
For adjuvant treatment of early breast cancer in postmenopausal women, the optimal duration of therapy is unknown. In the ATAC trial anastrozole was administered for five years. [see Clinical Studies (14.1)]
No dosage adjustment is necessary for patients with renal impairment or for elderly patients. [see Use in Specific Populations (8.6)]
2.2 Patients with Hepatic Impairment
No changes in dose are recommended for patients with mild-to-moderate hepatic impairment. Anastrozole has not been studied in patients with severe hepatic impairment. [see Use in Specific Populations (8.7)]
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Pregnancy and Premenopausal Women
Anastrozole may cause fetal harm when administered to a pregnant woman and offers no clinical benefit to premenopausal women with breast cancer. Anastrozole tablet is contraindicated in women who are or may become pregnant. There are no adequate and well-controlled studies in pregnant women using anastrozole tablets. If anastrozole tablet is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus or potential risk for loss of the pregnancy. [see Use in Specific Populations (8.1)]
4.2 Hypersensitivity
Anastrozole tablet is contraindicated in any patient who has shown a hypersensitivity reaction to the drug or to any of the excipients. Observed reactions include anaphylaxis, angioedema, and urticaria. [see Adverse Reactions (6.2)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Ischemic Cardiovascular Events
In women with pre-existing ischemic heart disease, an increased incidence of ischemic cardiovascular events was observed with anastrozole in the ATAC trial (17% of patients on anastrozole and 10% of patients on tamoxifen). Consider risk and benefits of anastrozole therapy in patients with pre-existing ischemic heart disease. [see Adverse Reactions (6.1)]
5.2 Bone Effects
Results from the ATAC trial bone substudy at 12 and 24 months demonstrated that patients receiving anastrozole had a mean decrease in both lumbar spine and total hip bone mineral density (BMD) compared to baseline. Patients receiving tamoxifen had a mean increase in both lumbar spine and total hip BMD compared to baseline [see Adverse Reactions, (6.1)].
5.3 Cholesterol
During the ATAC trial, more patients receiving anastrozole were reported to have an elevated serum cholesterol compared to patients receiving tamoxifen (9% versus 3.5%, respectively) [see Adverse Reactions, (6.1)].
-
6 ADVERSE REACTIONS
Serious adverse reactions with anastrozole occurring in less than 1 in 10,000 patients, are: 1) skin reactions such as lesions, ulcers, or blisters; 2) allergic reactions with swelling of the face, lips, tongue, and/or throat. This may cause difficulty in swallowing and/or breathing; and 3) changes in blood tests of the liver function, including inflammation of the liver with symptoms that may include a general feeling of not being well, with or without jaundice, liver pain or liver swelling [see Adverse Reactions (6.2)].
Common adverse reactions (occurring with an incidence of >10%) in women taking anastrozole included: hot flashes, asthenia, arthritis, pain, arthralgia, pharyngitis, hypertension, depression, nausea and vomiting, rash, osteoporosis, fractures, back pain, insomnia, pain, headache, bone pain, peripheral edema, increased cough, dyspnea, pharyngitis and lymphedema.
In the ATAC trial, the most common reported adverse reaction (>0.1%) leading to discontinuation of therapy for both treatment groups was hot flashes, although there were fewer patients who discontinued therapy as a result of hot flashes in the anastrozole group.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience
Adjuvant Therapy
Adverse reaction data for adjuvant therapy are based on the ATAC trial [see Clinical Studies (14.1)]. The median duration of adjuvant treatment for safety evaluation was 59.8 months and 59.6 months for patients receiving anastrozole 1 mg and tamoxifen 20 mg, respectively.
Adverse reactions occurring with an incidence of at least 5% in either treatment group during treatment or within 14 days of the end of treatment are presented in Table 1.
Table 1 - Adverse reactions occurring with an incidence of at least 5% in either treatment group during treatment, or within 14 days of the end of treatment in the ATAC trial* Body system and adverse reactions by
Anastrozole 1 mg
(N§ = 3092)
Tamoxifen 20 mg
(N§ = 3094)
- *
- The combination arm was discontinued due to lack of efficacy benefit at 33 months of follow-up.
- †
- COSTART Coding Symbols for Thesaurus of Adverse Reaction Terms.
- ‡
- A patient may have had more than 1 adverse reaction, including more than 1 adverse reaction in the same body system.
- §
- N=Number of patients receiving the treatment.
- ¶
- Vaginal Hemorrhage without further diagnosis.
Body as a whole
Asthenia
575 (19)
544 (18)
Pain
533 (17)
485 (16)
Back pain
321 (10)
309 (10)
Headache
314 (10)
249 (8)
Abdominal pain
271 (9)
276 (9)
Infection
285 (9)
276 (9)
Accidental injury
311 (10)
303 (10)
Flu syndrome
175 (6)
195 (6)
Chest pain
200 (7)
150 (5)
Neoplasm
162 (5)
144 (5)
Cyst
138 (5)
162 (5)
Cardiovascular
Vasodilatation
1104 (36)
1264 (41)
Hypertension
402 (13)
349 (11)
Digestive
Nausea
343 (11)
335 (11)
Constipation
249 (8)
252 (8)
Diarrhea
265 (9)
216 (7)
Dyspepsia
206 (7)
169 (6)
Gastrointestinal disorder
210 (7)
158 (5)
Hemic and lymphatic
Lymphedema
304 (10)
341 (11)
Anemia
113 (4)
159 (5)
Metabolic and nutritional
Peripheral edema
311 (10)
343 (11)
Weight gain
285 (9)
274 (9)
Hypercholesterolemia
278 (9)
108 (3.5)
Musculoskeletal
Arthritis
512 (17)
445 (14)
Arthralgia
467 (15)
344 (11)
Osteoporosis
325 (11)
226 (7)
Fracture
315 (10)
209 (7)
Bone pain
201 (7)
185 (6)
Arthrosis
207 (7)
156 (5)
Joint Disorder
184 (6)
160 (5)
Myalgia
179 (6)
160 (5)
Nervous system
Depression
413 (13)
382 (12)
Insomnia
309 (10)
281 (9)
Dizziness
236 (8)
234 (8)
Anxiety
195 (6)
180 (6)
Paresthesia
215 (7)
145 (5)
Respiratory
Pharyngitis
443 (14)
422 (14)
Cough increased
261 (8)
287 (9)
Dyspnea
234 (8)
237 (8)
Sinusitis
184 (6)
159 (5)
Bronchitis
167 (5)
153 (5)
Skin and appendages
Rash
333 (11)
387 (13)
Sweating
145 (5)
177 (6)
Special Senses
Cataract Specified
182 (6)
213 (7)
Urogenital
Leukorrhea
86 (3)
286 (9)
Urinary tract infection
244 (8)
313 (10)
Breast pain
251 (8)
169 (6)
Breast Neoplasm
164 (5)
139 (5)
Vulvovaginitis
194 (6)
150 (5)
Vaginal Hemorrhage¶
122 (4)
180 (6)
Vaginitis
125 (4)
158 (5)
Certain adverse reactions and combinations of adverse reactions were prospectively specified for analysis, based on the known pharmacologic properties and side effect profiles of the two drugs (see Table 2).
Table 2 — Number of Patients with Pre-specified Adverse Reactions in ATAC Trial* Anastrozole
N=3092
(%)
Tamoxifen
N=3094
(%)
Odds-ratio
95% CI
Hot Flashes
1104 (36)
1264 (41)
0.80
0.73 to 0.89
Musculoskeletal Events†
1100 (36)
911 (29)
1.32
1.19 to 1.47
Fatigue/Asthenia
575 (19)
544 (18)
1.07
0.94 to 1.22
Mood Disturbances
597 (19)
554 (18)
1.10
0.97 to 1.25
Nausea and Vomiting
393 (13)
384 (12)
1.03
0.88 to 1.19
All Fractures
315 (10)
209 (7)
1.57
1.30 to 1.88
Fractures of Spine, Hip, or Wrist
133 (4)
91 (3)
1.48
1.13 to 1.95
Wrist/Colles’ fractures
67 (2)
50 (2)
Spine fractures
43 (1)
22 (1)
Hip fractures
28 (1)
26 (1)
Cataracts
182 (6)
213 (7)
0.85
0.69 to 1.04
Vaginal Bleeding
167 (5)
317 (10)
0.50
0.41 to 0.61
Ischemic Cardiovascular Disease
127 (4)
104 (3)
1.23
0.95 to 1.60
Vaginal Discharge
109 (4)
408 (13)
0.24
0.19 to 0.30
Venous Thromboembolic events
87 (3)
140 (5)
0.61
0.47 to 0.80
Deep Venous Thromboembolic Events
48 (2)
74 (2)
0.64
0.45 to 0.93
Ischemic Cerebrovascular Event
62 (2)
88 (3)
0.70
0.50 to 0.97
Endometrial Cancer‡
4 (0.2)
13 (0.6)
0.31
0.10 to 0.94
Ischemic Cardiovascular Events
Between treatment arms in the overall population of 6186 patients, there was no statistical difference in ischemic cardiovascular events (4% anastrozole vs. 3% tamoxifen). In the overall population, angina pectoris was reported in 71/3092 (2.3%) patients in the anastrozole arm and 51/3094 (1.6%) patients in the tamoxifen arm; myocardial infarction was reported in 37/3092 (1.2%) patients in the anastrozole arm and 34/3094 (1.1%) patients in the tamoxifen arm.
In women with pre-existing ischemic heart disease 465/6186 (7.5%), the incidence of ischemic cardiovascular events was 17% in patients on anastrozole and 10% in patients on tamoxifen. In this patient population, angina pectoris was reported in 25/216 (11.6%) patients receiving anastrozole and 13/249 (5.2%) patients receiving tamoxifen; myocardial infarction was reported in 2/216 (0.9%) patients receiving anastrozole and 8/249 (3.2%) patients receiving tamoxifen.
Bone Mineral Density Findings
Results from the ATAC trial bone substudy at 12 and 24 months demonstrated that patients receiving anastrozole had a mean decrease in both lumbar spine and total hip bone mineral density (BMD) compared to baseline. Patients receiving tamoxifen had a mean increase in both lumbar spine and total hip BMD compared to baseline.
Because anastrozole lowers circulating estrogen levels it may cause a reduction in bone mineral density.
A post-marketing trial assessed the combined effects of anastrozole and the bisphosphonate risedronate on changes from baseline in BMD and markers of bone resorption and formation in postmenopausal women with hormone receptor-positive early breast cancer. All patients received calcium and vitamin D supplementation. At 12 months, small reductions in lumbar spine bone mineral density were noted in patients not receiving bisphosphonates. Bisphosphonate treatment preserved bone density in most patients at risk of fracture.
Postmenopausal women with early breast cancer scheduled to be treated with anastrozole should have their bone status managed according to treatment guidelines already available for postmenopausal women at similar risk of fragility fracture.
Cholesterol
During the ATAC trial, more patients receiving anastrozole were reported to have an elevated serum cholesterol compared to patients receiving tamoxifen (9% versus 3.5%, respectively).
A post-marketing trial also evaluated any potential effects of anastrozole on lipid profile. In the primary analysis population for lipids (anastrozole alone), there was no clinically significant change in LDL-C from baseline to 12 months and HDL-C from baseline to 12 months
In secondary population for lipids (anastrozole+risedronate), there also was no clinically significant change in LDL-C and HDL-C from baseline to 12 months.
In both populations for lipids, there was no clinically significant difference in total cholesterol (TC) or serum triglycerides (TG) at 12 months compared with baseline.
In this trial, treatment for 12 months with anastrozole alone had a neutral effect on lipid profile. Combination treatment with anastrozole and risedronate also had a neutral effect on lipid profile.
The trial provides evidence that postmenopausal women with early breast cancer scheduled to be treated with anastrozole should be managed using the current National Cholesterol Education Program guidelines for cardiovascular risk-based management of individual patients with LDL elevations.
Other Adverse Reactions
Patients receiving anastrozole had an increase in joint disorders (including arthritis, arthrosis and arthralgia) compared with patients receiving tamoxifen. Patients receiving anastrozole had an increase in the incidence of all fractures (specifically fractures of spine, hip and wrist) [315 (10%)] compared with patients receiving tamoxifen [209 (7%)].
Patients receiving anastrozole had a higher incidence of carpal tunnel syndrome [78 (2.5%)] compared with patients receiving tamoxifen [22 (0.7%)].
Vaginal bleeding occurred more frequently in the tamoxifen-treated patients versus the anastrozole-treated patients 317 (10%) versus 167 (5%), respectively.
Patients receiving anastrozole had a lower incidence of hot flashes, vaginal bleeding, vaginal discharge, endometrial cancer, venous thromboembolic events and ischemic cerebrovascular events compared with patients receiving tamoxifen.
First-Line Therapy
Adverse reactions occurring with an incidence of at least 5% in either treatment group of trials 0030 and 0027 during or within 2 weeks of the end of treatment are shown in Table 3.
Table 3 — Adverse Reactions Occurring with an Incidence of at Least 5% in Trials 0030 and 0027 Body system
Adverse Reaction*
Number (%) of subjects
Anastrozole
(n=506)
Tamoxifen
(n=511)
- *
- A patient may have had more than 1 adverse event.
Whole body
Asthenia
83 (16)
81 (16)
Pain
70 (14)
73 (14)
Back pain
60 (12)
68 (13)
Headache
47 (9)
40 (8)
Abdominal pain
40 (8)
38 (7)
Chest pain
37 (7)
37 (7)
Flu syndrome
35 (7)
30 (6)
Pelvic pain
23 (5)
30 (6)
Cardiovascular
Vasodilation
128 (25)
106 (21)
Hypertension
25 (5)
36 (7)
Digestive
Nausea
94 (19)
106 (21)
Constipation
47 (9)
66 (13)
Diarrhea
40 (8)
33 (6)
Vomiting
38 (8)
36 (7)
Anorexia
26 (5)
46 (9)
Metabolic and Nutritional
Peripheral edema
51 (10)
41 (8)
Muscoloskeletal
Bone pain
54 (11)
52 (10)
Nervous
Dizziness
30 (6)
22 (4)
Insomnia
30 (6)
38 (7)
Depression
23 (5)
32 (6)
Hypertonia
16 (3)
26 (5)
Respiratory
Cough increased
55 (11)
52 (10)
Dyspnea
51 (10)
47 (9)
Pharyngitis
49 (10)
68 (13)
Skin and appendages
Rash
38 (8)
34 (8)
Urogenital
Leukorrhea
9 (2)
31 (6)
Less frequent adverse experiences reported in patients receiving anastrozole 1 mg in either Trial 0030 or Trial 0027 were similar to those reported for second-line therapy.
Based on results from second-line therapy and the established safety profile of tamoxifen, the incidences of 9 pre-specified adverse event categories potentially causally related to one or both of the therapies because of their pharmacology were statistically analyzed. No significant differences were seen between treatment groups.
Table 4 — Number of Patients with Pre-specified Adverse Reactions in Trials 0030 and 0027 Number (n) and Percentage of Patients
Adverse Reaction*
Anastrozole
1 mg
(n=506)
n (%)
NOLVADEX
20 mg
(n=511)
n (%)
Depression
23 (5)
32 (6)
Tumor Flare
15 (3)
18 (4)
Thromboembolic Disease†
18 (4)
33 (6)
Venous†
5
15
Coronary and Cerebral‡
13
19
Gastrointestinal Disturbance
170 (34)
196 (38)
Hot Flushes
134 (26)
118 (23)
Vaginal Dryness
9 (2)
3 (1)
Lethargy
6 (1)
15 (3)
Vaginal Bleeding
5 (1)
11 (2)
Weight Gain
11 (2)
8 (2)
Second-Line Therapy
Anastrozole was tolerated in two controlled clinical trials (i.e., Trials 0004 and 0005), with less than 3.3% of the anastrozole-treated patients and 4.0% of the megestrol acetate-treated patients withdrawing due to an adverse reaction.
The principal adverse reaction more common with anastrozole than megestrol acetate was diarrhea. Adverse reactions reported in greater than 5% of the patients in any of the treatment groups in these two well-controlled clinical trials, regardless of causality, are presented below:
Table 5 — Number (N) and Percentage of Patients with Adverse Reactions in Trials 0004 and 0005* Adverse Reaction
Anastrozole
Anastrozole
Megesterol
Acetate
1 mg
10 mg
160 mg
(n=262)
(n=246)
(n=253)
n
%
n
%
n
%
- *
- A patient may have had more then one adverse reaction
Asthenia
42
(16)
33
(13)
47
(19)
Nausea
41
(16)
48
(20)
28
(11)
Headache
34
(13)
44
(18)
24
(9)
Hot Flashes
32
(12)
29
(11)
21
(8)
Pain
28
(11)
38
(15)
29
(11)
Back Pain
28
(11)
26
(11)
19
(8)
Dyspnea
24
(9)
27
(11)
53
(21)
Vomiting
24
(9)
26
(11)
16
(6)
Cough Increased
22
(8)
18
(7)
19
(8)
Diarrhea
22
(8)
18
(7)
7
(3)
Constipation
18
(7)
18
(7)
21
(8)
Abdominal Pain
18
(7)
14
(6)
18
(7)
Anorexia
18
(7)
19
(8)
11
(4)
Bone Pain
17
(6)
26
(12)
19
(8)
Pharyngitis
16
(6)
23
(9)
15
(6)
Dizziness
16
(6)
12
(5)
15
(6)
Rash
15
(6)
15
(6)
19
(8)
Dry Mouth
15
(6)
11
(4)
13
(5)
Peripheral Edema
14
(5)
21
(9)
28
(11)
Pelvic Pain
14
(5)
17
(7)
13
(5)
Depression
14
(5)
6
(2)
5
(2)
Chest Pain
13
(5)
18
(7)
13
(5)
Paresthesia
12
(5)
15
(6)
9
(4)
Vaginal Hemorrhage
6
(2)
4
(2)
13
(5)
Weight Gain
4
(2)
9
(4)
30
(12)
Sweating
4
(2)
3
(1)
16
(6)
Increased Appetite
0
(0)
1
(0)
13
(5)
Other less frequent (2% to 5%) adverse reactions reported in patients receiving anastrozole 1 mg in either Trial 0004 or Trial 0005 are listed below. These adverse experiences are listed by body system and are in order of decreasing frequency within each body system regardless of assessed causality.
Body as a Whole: Flu syndrome; fever; neck pain; malaise; accidental injury; infection
Cardiovascular: Hypertension; thrombophlebitis
Hepatic: Gamma GT increased; SGOT increased; SGPT increased
Hematologic: Anemia; leukopenia
Metabolic and Nutritional: Alkaline phosphatase increased; weight loss
Mean serum total cholesterol levels increased by 0.5 mmol/L among patients receiving anastrozole. Increases in LDL cholesterol have been shown to contribute to these changes.
Musculoskeletal: Myalgia; arthralgia; pathological fracture
Nervous: Somnolence; confusion; insomnia; anxiety; nervousness
Respiratory: Sinusitis; bronchitis; rhinitis
Skin and Appendages: Hair thinning (alopecia); pruritus
Urogenital: Urinary tract infection; breast pain
The incidences of the following adverse event groups potentially causally related to one or both of the therapies because of their pharmacology, were statistically analyzed: weight gain, edema, thromboembolic disease, gastrointestinal disturbance, hot flushes, and vaginal dryness. These six groups, and the adverse reactions captured in the groups, were prospectively defined. The results are shown in the table below.
Table 6 — Number (n) and Percentage of Patients with Pre-specified Adverse Reactions in Trials 0004 and 0005 Anastrozole
Anastrozole
Megestrol Acetate
1 mg
10 mg
160 mg
(n=262)
(n=246)
(n=253)
Adverse Event Group
n
(%)
n
(%)
n
(%)
Gastrointestinal Disturbance
77
(29)
81
(33)
54
(21)
Hot Flushes
33
(13)
29
(12)
35
(14)
Edema
19
(7)
28
(11)
35
(14)
Thromboembolic Disease
9
(3)
4
(2)
12
(5)
Vaginal Dryness
5
(2)
3
(1)
2
(1)
Weight Gain
4
(2)
10
(4)
30
(12)
6.2 Post-Marketing Experience
Hepatobiliary events including increases in alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase have been reported (≤1% and <10%) and gamma-GT, bilirubin and hepatitis have been reported (≤0.1% and <1%) in patients receiving anastrozole.
Anastrozole may also be associated with rash including cases of mucocutaneous disorders such as erythema multiforme and Stevens-Johnson syndrome.
Cases of allergic reactions including angioedema, urticaria and anaphylaxis have been reported in patients receiving anastrozole. [see Contraindications (4.2)]
Trigger finger has been reported (>0.1% and <1%) in patients receiving anastrozole.
-
7 DRUG INTERACTIONS
7.1 Tamoxifen
Co-administration of anastrozole and tamoxifen in breast cancer patients reduced anastrozole plasma concentration by 27%. However, the coadministration did not affect the pharmacokinetics of tamoxifen or N-desmethyltamoxifen. At a median follow-up of 33 months, the combination of anastrozole and tamoxifen did not demonstrate any efficacy benefit when compared with tamoxifen in all patients as well as in the hormone receptor-positive subpopulation. This treatment arm was discontinued from the trial. [see Clinical Studies (14.1)]. Based on clinical and pharmacokinetic results from the ATAC trial, tamoxifen should not be administered with anastrozole.
7.2 Estrogen
Estrogen-containing therapies should not be used with anastrozole as they may diminish its pharmacological action.
7.3 Warfarin
In a study conducted in 16 male volunteers, anastrozole did not alter the exposure (as measured by Cmax and AUC), and anticoagulant activity (as measured by prothrombin time, activated partial thromboplastin time, and thrombin time) of both R- and S-warfarin.
7.4 Cytochrome P450
Based on these in vitro and in vivo results, it is unlikely that co-administration of anastrozole 1 mg will affect other drugs as a result inhibition of cytochrome P450 [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
PREGNANCY CATEGORY X [see Contraindications (4.1)]
Anastrozole may cause fetal harm when administered to a pregnant woman and offers no clinical benefit to premenopausal women with breast cancer. Anastrozole is contraindicated in women who are or may become pregnant. In animal studies, anastrozole caused pregnancy failure, increased pregnancy loss, and signs of delayed fetal development. There are no studies of anastrozole use in pregnant women. If anastrozole is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus and potential risk for pregnancy loss.
In animal reproduction studies, pregnant rats and rabbits received anastrozole during organogenesis at doses equal to or greater than 1 (rats) and 1/3 (rabbits) the recommended human dose on a mg/m2 basis. In both species, anastrozole crossed the placenta, and there was increased pregnancy loss (increased pre- and/or post-implantation loss, increased resorption, and decreased numbers of live fetuses). In rats, these effects were dose related, and placental weights were significantly increased. Fetotoxicity, including delayed fetal development (i.e., incomplete ossification and depressed fetal body weights), occurred in rats at anastrozole doses that produced peak plasma levels 19 times higher than serum levels in humans at the therapeutic dose (AUC0-24hr 9 times higher). In rabbits, anastrozole caused pregnancy failure at doses equal to or greater than 16 times the recommended human dose on a mg/m2 basis. [see Animal Toxicology and/or Pharmacology (13.2)]
8.3 Nursing Mothers
It is not known if anastrozole is excreted in human milk. Because many drugs are excreted in human milk and because of the tumorigenicity shown for anastrozole in animal studies, or the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The efficacy of anastrozole tablets in the treatment of pubertal gynecomastia in adolescent boys and in the treatment of precocious puberty in girls with McCune-Albright Syndrome has not been demonstrated.
Labeling describing clinical trials and pharmacokinetic studies of anastrozole in pubertal boys of adolescent age with gynecomastia and in girls with McCune-Albright Syndrome and progressive precocious puberty is approved for AstraZeneca Pharmaceuticals LP’s Arimidex®. However, due to AstraZeneca Pharmaceuticals LP’s marketing exclusivity rights, a description of those trials and studies is not approved for this anastrozole labeling.
8.5 Geriatric Use
In studies 0030 and 0027 about 50% of patients were 65 or older. Patients ≥ 65 years of age had moderately better tumor response and time to tumor progression than patients < 65 years of age regardless of randomized treatment. In studies 0004 and 0005 50% of patients were 65 or older. Response rates and time to progression were similar for the over 65 and younger patients.
In the ATAC study 45% of patients were 65 years of age or older. The efficacy of anastrozole compared to tamoxifen in patients who were 65 years or older (N=1413 for anastrozole and N=1410 for tamoxifen, the hazard ratio for disease-free survival was 0.93 (95% CI: 0.80, 1.08)) was less than efficacy observed in patients who were less than 65 years of age (N=1712 for anastrozole and N=1706 for tamoxifen, the hazard ratio for disease-free survival was 0.79 (95% CI: 0.67, 0.94)).
The pharmacokinetics of anastrozole are not affected by age.
8.6 Renal Impairment
Since only about 10% of anastrozole is excreted unchanged in the urine, the renal impairment does not influence the total body clearance. Dosage adjustment in patients with renal impairment is not necessary [see Dosage and Administration (2.1) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
The plasma anastrozole concentrations in the subjects with hepatic cirrhosis were within the range of concentrations seen in normal subjects across all clinical trials. Therefore, dosage adjustment is also not necessary in patients with stable hepatic cirrhosis. Anastrozole has not been studied in patients with severe hepatic impairment [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Clinical trials have been conducted with anastrozole, up to 60 mg in a single dose given to healthy male volunteers and up to 10 mg daily given to postmenopausal women with advanced breast cancer; these dosages were tolerated. A single dose of anastrozole that results in life-threatening symptoms has not been established. There is no specific antidote to overdosage and treatment must be symptomatic. In the management of an overdose, consider that multiple agents may have been taken. Vomiting may be induced if the patient is alert. Dialysis may be helpful because anastrozole is not highly protein bound. General supportive care, including frequent monitoring of vital signs and close observation of the patient, is indicated.
-
11 DESCRIPTION
Anastrozole tablets for oral administration contain 1 mg of anastrozole, a non-steroidal aromatase inhibitor. It is chemically described as 1,3-Benzenediacetonitrile, a, a, a', a'-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl). Its molecular formula is C17H19N5 and its structural formula is:
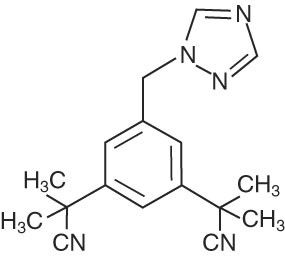
Anastrozole is an off-white powder with a molecular weight of 293.4. Anastrozole has moderate aqueous solubility (0.5 mg/mL at 25°C); solubility is independent of pH in the physiological range. Anastrozole is freely soluble in methanol, acetone, ethanol, and tetrahydrofuran, and very soluble in acetonitrile.
Each tablet contains as inactive ingredients: lactose monohydrate, magnesium stearate, hypromellose, macrogol, povidone, sodium starch glycolate, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The growth of many cancers of the breast is stimulated or maintained by estrogens. Treatment of breast cancer thought to be hormonally responsive (i.e., estrogen and/or progesterone receptor positive or receptor unknown) has included a variety of efforts to decrease estrogen levels (ovariectomy, adrenalectomy, hypophysectomy) or inhibit estrogen effects (antiestrogens and progestational agents). These interventions lead to decreased tumor mass or delayed progression of tumor growth in some women.
In postmenopausal women, estrogens are mainly derived from the action of the aromatase enzyme, which converts adrenal androgens (primarily androstenedione and testosterone) to estrone and estradiol. The suppression of estrogen biosynthesis in peripheral tissues and in the cancer tissue itself can therefore be achieved by specifically inhibiting the aromatase enzyme.
Anastrozole is a potent and selective non-steroidal aromatase inhibitor. It significantly lowers serum estradiol concentrations and has no detectable effect on formation of adrenal corticosteroids or aldosterone.
12.2 Pharmacodynamics
Effect on Estradiol
Mean serum concentrations of estradiol were evaluated in multiple daily dosing trials with 0.5, 1, 3, 5, and 10 mg of anastrozole in postmenopausal women with advanced breast cancer. Clinically significant suppression of serum estradiol was seen with all doses. Doses of 1 mg and higher resulted in suppression of mean serum concentrations of estradiol to the lower limit of detection (3.7 pmol/L). The recommended daily dose, anastrozole 1 mg, reduced estradiol by approximately 70% within 24 hours and by approximately 80% after 14 days of daily dosing. Suppression of serum estradiol was maintained for up to 6 days after cessation of daily dosing with anastrozole 1 mg.
The effect of anastrozole in premenopausal women with early or advanced breast cancer has not been studied. Because aromatization of adrenal androgens is not a significant source of estradiol in premenopausal women, anastrozole would not be expected to lower estradiol levels in premenopausal women.
Effect on Corticosteroids
In multiple daily dosing trials with 3, 5, and 10 mg, the selectivity of anastrozole was assessed by examining effects on corticosteroid synthesis. For all doses, anastrozole did not affect cortisol or aldosterone secretion at baseline or in response to ACTH. No glucocorticoid or mineralocorticoid replacement therapy is necessary with anastrozole.
Other Endocrine Effects
In multiple daily dosing trials with 5 and 10 mg, thyroid stimulating hormone (TSH) was measured; there was no increase in TSH during the administration of anastrozole. Anastrozole does not possess direct progestogenic, androgenic, or estrogenic activity in animals, but does perturb the circulating levels of progesterone, androgens, and estrogens.
12.3 Pharmacokinetics
Absorption
Inhibition of aromatase activity is primarily due to anastrozole, the parent drug. Absorption of anastrozole is rapid and maximum plasma concentrations typically occur within 2 hours of dosing under fasted conditions. Studies with radiolabeled drug have demonstrated that orally administered anastrozole is well absorbed into the systemic circulation. Food reduces the rate but not the overall extent of anastrozole absorption. The mean Cmax of anastrozole decreased by 16% and the median Tmax was delayed from 2 to 5 hours when anastrozole was administered 30 minutes after food. The pharmacokinetics of anastrozole are linear over the dose range of 1 to 20 mg, and do not change with repeated dosing. The pharmacokinetics of anastrozole were similar in patients and healthy volunteers.
Distribution
Steady-state plasma levels are approximately 3- to 4-fold higher than levels observed after a single dose of anastrozole. Plasma concentrations approach steady-state levels at about 7 days of once daily dosing. Anastrozole is 40% bound to plasma proteins in the therapeutic range.
Metabolism
Metabolism of anastrozole occurs by N-dealkylation, hydroxylation and glucuronidation. Three metabolites of anastrozole (triazole, a glucuronide conjugate of hydroxy-anastrozole, and a glucuronide conjugate of anastrozole itself) have been identified in human plasma and urine. The major circulating metabolite of anastrozole, triazole, lacks pharmacologic activity.
Anastrozole inhibited reactions catalyzed by cytochrome P450 1A2, 2C8/9, and 3A4 in vitro with Ki values which were approximately 30 times higher than the mean steady-state Cmax values observed following a 1 mg daily dose. Anastrozole had no inhibitory effect on reactions catalyzed by cytochrome P450 2A6 or 2D6 in vitro. Administration of a single 30 mg/kg or multiple 10 mg/kg doses of anastrozole to healthy subjects had no effect on the clearance of antipyrine or urinary recovery of antipyrine metabolites.
Excretion
Eighty-five percent of radiolabeled anastrozole was recovered in feces and urine. Hepatic metabolism accounts for approximately 85% of anastrozole elimination. Renal elimination accounts for approximately 10% of total clearance. The mean elimination half-life of anastrozole is 50 hours.
Effect of Gender and Age
Anastrozole pharmacokinetics have been investigated in postmenopausal female volunteers and patients with breast cancer. No age related effects were seen over the range <50 to >80 years.
Effect of Race
Estradiol and estrone sulfate serum levels were similar between Japanese and Caucasian postmenopausal women who received 1 mg of anastrozole daily for 16 days. Anastrozole mean steady-state minimum plasma concentrations in Caucasian and Japanese postmenopausal women were 25.7 and 30.4 ng/mL, respectively.
Effect of Renal Impairment
Anastrozole pharmacokinetics have been investigated in subjects with renal impairment. Anastrozole renal clearance decreased proportionally with creatinine clearance and was approximately 50% lower in volunteers with severe renal impairment (creatinine clearance < 30 mL/min/1.73m2) compared to controls. Total clearance was only reduced 10%. No dosage adjustment is needed for renal impairment. [see Dosage and Administration (2.1) and Use in Specific Populations (8.6)]
Effect of Hepatic Impairment
Anastrozole pharmacokinetics have been investigated in subjects with hepatic cirrhosis related to alcohol abuse. The apparent oral clearance (CL/F) of anastrozole was approximately 30% lower in subjects with stable hepatic cirrhosis than in control subjects with normal liver function. However, these plasma concentrations were still with the range of values observed in normal subjects. The effect of severe hepatic impairment was not studied. No dose adjustment is necessary for stable hepatic cirrhosis. [see Dosage and Administration (2.2) and Use in Specific Populations (8.7)]
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A conventional carcinogenesis study in rats at doses of 1.0 to 25 mg/kg/day (about 10 to 243 times the daily maximum recommended human dose on a mg/m2 basis) administered by oral gavage for up to 2 years revealed an increase in the incidence of hepatocellular adenoma and carcinoma and uterine stromal polyps in females and thyroid adenoma in males at the high dose. A dose related increase was observed in the incidence of ovarian and uterine hyperplasia in females. At 25 mg/kg/day, plasma AUC0-24 hr levels in rats were 110 to 125 times higher than the level exhibited in postmenopausal volunteers at the recommended dose. A separate carcinogenicity study in mice at oral doses of 5 to 50 mg/kg/day (about 24 to 243 times the daily maximum recommended human dose on a mg/m2 basis) for up to 2 years produced an increase in the incidence of benign ovarian stromal, epithelial and granulosa cell tumors at all dose levels. A dose related increase in the incidence of ovarian hyperplasia was also observed in female mice. These ovarian changes are considered to be rodent-specific effects of aromatase inhibition and are of questionable significance to humans. The incidence of lymphosarcoma was increased in males and females at the high dose. At 50 mg/kg/day, plasma AUC levels in mice were 35 to 40 times higher than the level exhibited in postmenopausal volunteers at the recommended dose.
Anastrozole has not been shown to be mutagenic in in vitro tests (Ames and E. coli bacterial tests, CHO-K1 gene mutation assay) or clastogenic either in vitro (chromosome aberrations in human lymphocytes) or in vivo (micronucleus test in rats).
Oral administration of anastrozole to female rats (from 2 weeks before mating to pregnancy day 7) produced significant incidence of infertility and reduced numbers of viable pregnancies at 1 mg/kg/day (about 10 times the recommended human dose on a mg/m2 basis and 9 times higher than the AUC0-24 hr found in postmenopausal volunteers at the recommended dose). Pre-implantation loss of ova or fetus was increased at doses equal to or greater than 0.02 mg/kg/day (about one-fifth the recommended human dose on a mg/m2 basis). Recovery of fertility was observed following a 5-week non-dosing period which followed 3 weeks of dosing. It is not known whether these effects observed in female rats are indicative of impaired fertility in humans.
Multiple-dose studies in rats administered anastrozole for 6 months at doses equal to or greater than 1 mg/kg/day (which produced plasma anastrozole Cssmax and AUC0-24 hr that were 19 and 9 times higher than the respective values found in postmenopausal volunteers at the recommended dose) resulted in hypertrophy of the ovaries and the presence of follicular cysts. In addition, hyperplastic uteri were observed in 6-month studies in female dogs administered doses equal to or greater than 1 mg/kg/day (which produced plasma anastrozole Cssmax and AUC0-24 hr that were 22 times and 16 times higher than the respective values found in postmenopausal women at the recommended dose). It is not known whether these effects on the reproductive organs of animals are associated with impaired fertility in premenopausal women.
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology
Anastrozole has been found to cross the placenta following oral administration of 0.1 mg/kg in rats and rabbits (about 1 and 1.9 times the recommended human dose, respectively, on a mg/m2 basis). Studies in both rats and rabbits at doses equal to or greater than 0.1 and 0.02 mg/kg/day, respectively (about 1 and 1/3, respectively, the recommended human dose on a mg/m2 basis), administered during the period of organogenesis showed that anastrozole increased pregnancy loss (increased pre- and/or post-implantation loss, increased resorption, and decreased numbers of live fetuses); effects were dose related in rats. Placental weights were significantly increased in rats at doses of 0.1 mg/kg/day or more.
Evidence of fetotoxicity, including delayed fetal development (i.e., incomplete ossification and depressed fetal body weights), was observed in rats administered doses of 1 mg/kg/day (which produced plasma anastrozole Cssmax and AUC0-24 hr that were 19 times and 9 times higher than the respective values found in postmenopausal volunteers at the recommended dose). There was no evidence of teratogenicity in rats administered doses up to 1.0 mg/kg/day. In rabbits, anastrozole caused pregnancy failure at doses equal to or greater than 1.0 mg/kg/day (about 16 times the recommended human dose on a mg/m2 basis); there was no evidence of teratogenicity in rabbits administered 0.2 mg/kg/day (about 3 times the recommended human dose on a mg/m2 basis).
-
14 CLINICAL STUDIES
14.1 Adjuvant Treatment of Breast Cancer in Postmenopausal Women
A multicenter, double-blind trial (ATAC) randomized 9,366 postmenopausal women with operable breast cancer to adjuvant treatment with anastrozole 1 mg daily, tamoxifen 20 mg daily, or a combination of the two treatments for five years or until recurrence of the disease.
The primary endpoint of the trial was disease-free survival (ie, time to occurrence of a distant or local recurrence, or contralateral breast cancer or death from any cause). Secondary endpoints of the trial included distant disease-free survival, the incidence of contralateral breast cancer and overall survival. At a median follow-up of 33 months, the combination of anastrozole and tamoxifen did not demonstrate any efficacy benefit when compared with tamoxifen in all patients as well as in the hormone receptor positive subpopulation. This treatment arm was discontinued from the trial. Based on clinical and pharmacokinetic results from the ATAC trial, tamoxifen should not be administered with anastrozole. [see Drug Interactions (7.1)]
Demographic and other baseline characteristics were similar among the three treatment groups (see Table 7).
Table 7 - Demographic and Baseline Characteristics for ATAC Trial Demographic Characteristic
Anastrozole
1 mg
(N*=3125)
Tamoxifen
20 mg
(N*=3116)
Anastrozole 1 mg
plus Tamoxifen
20 mg†
(N*=3125)
- *
- N=Number of patients randomized to the treatment
- †
- The combination arm was discontinued due to lack of efficacy benefit at 33 months of follow-up
- ‡
- Includes patients who were estrogen receptor (ER) positive or progesterone receptor (PgR) positive, or both positive
- §
- Includes patients with both ER negative and PgR negative receptor status
- ¶
- Includes all other combinations of ER and PgR receptor status unknown
- #
- Among the patients who had breast conservation, radiotherapy was administered to 95.0% of patients in the anastrozole arm, 94.1% in the tamoxifen arm and 94.5% in the anastrozole plus tamoxifen arm.
Mean age (yrs.)
64.1
64.1
64.3
Age Range (yrs.)
38.1 to 92.8
32.8 to 94.9
37 to 92.2
Age Distribution (%)
<45 yrs.
0.7
0.4
0.5
45 to 60 yrs.
34.6
35.0
34.5
>60 <70 yrs.
38.0
37.1
37.7
>70 yrs.
26.7
27.4
27.3
Mean Weight (kg)
70.8
71.1
71.3
Receptor Status (%)
Positive‡
83.5
83.1
84.0
Negative§
7.4
8.0
7.0
Other¶
8.8
8.6
9.0
Other Treatment (%) prior to Randomization
Mastectomy
47.8
47.3
48.1
Breast conservation#
52.3
52.8
51.9
Axillary surgery
95.5
95.7
95.2
Radiotherapy
63.3
62.5
61.9
Chemotherapy
22.3
20.8
20.8
Neoadjuvant Tamoxifen
1.6
1.6
1.7
Primary Tumor Size (%)
T1 (≤2 cm)
63.9
62.9
64.1
T2 (>2 cm and ≤5 cm)
32.6
34.2
32.9
T3 (>5 cm)
2.7
2.2
2.3
Nodal Status (%)
Node positive
34.9
33.6
33.5
1 to 3 (# of nodes)
24.4
24.4
24.3
4 to 9
7.5
6.4
6.8
>9
2.9
2.7
2.3
Tumor Grade (%)
Well-differentiated
20.8
20.5
21.2
Moderately differentiated
46.8
47.8
46.5
Poorly/undifferentiated
23.7
23.3
23.7
Not assessed/recorded
8.7
8.4
8.5
Patients in the two monotherapy arms of the ATAC trial were treated for a median of 60 months (5 years) and followed for a median of 68 months. Disease-free survival in the intent-to-treat population was statistically significantly improved [Hazard Ratio (HR) = 0.87, 95% CI: 0.78, 0.97, p=0.0127 in the anastrozole arm compared to the tamoxifen arm. In the hormone receptor-positive subpopulation representing about 84% of the trial patients, disease-free survival was also statistically significantly improved (HR =0.83, 95% CI: 0.73, 0.94, p=0.0049) in the anastrozole arm compared to the tamoxifen arm.
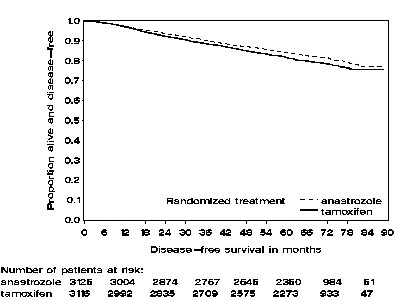
Figure 1 — Disease-Free Survival Kaplan Meier Survival Curve for all Patients Randomized to anastrozole or Tamoxifen Monotherapy in the ATAC trial (Intent-to-Treat)
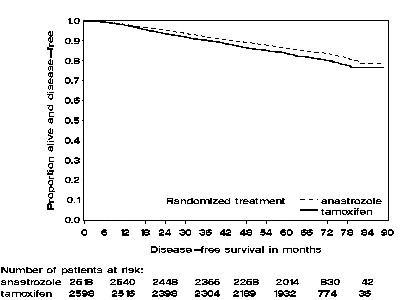
Figure 2 — Disease-free Survival for Hormone Receptor-Positive Subpopulation of Patients Randomized to anastrozole or Tamoxifen Monotherapy in the ATAC Trial
The survival data with 68 months follow-up is presented in Table 9.
In the group of patients who had previous adjuvant chemotherapy (N=698 for anastrozole and N=647 for tamoxifen), the hazard ratio for disease-free survival was 0.91 (95% CI: 0.73 to 1.13) in the anastrozole arm compared to the tamoxifen arm.
The frequency of individual events in the intent-to-treat population and the hormone receptor-positive subpopulation are described in Table 8.
Table 8 - All Recurrence and Death Events* Intent-To-Treat Population†
Hormone Receptor-Positive Subpopulation†
Anastrozole
1 mg
(N‡=3125)
Tamoxifen
20 mg
(N‡=3116)
Anastrozole
1 mg
(N‡=2618)
Tamoxifen
20 mg
(N‡=2598
Number (%) of Patients
Number (%) of Patients
Median Duration of Therapy (mo)
60
60
60
60
Median Efficacy
Follow-up (mo)
68
68
68
68
Loco-regional recurrence
119 (3.8)
149 (4.8)
76 (2.9)
101 (3.9)
Contralateral breast cancer
35 (1.1)
59 (1.9)
26 (1.0)
54 (2.1)
Invasive
27 (0.9)
52 (1.7)
21 (0.8)
48 (1.8)
Ductal carcinoma
in situ
8 (0.3)
6 (0.2)
5 (0.2)
5 (0.2)
Unknown
0
1 (<0.1)
0
1 (<0.1)
Distant recurrence
324 (10.4)
375 (12.0)
226 (8.6)
265 (10.2)
Death from Any Cause
411 (13.2)
420 (13.5)
296 (11.3)
301 (11.6)
Death breast cancer
218 (7.0)
248 (8.0)
138 (5.3)
160 (6.2)
Death other reason
(including unknown)
193 (6.2)
172 (5.5)
158 (6.0)
141 (5.4)
A summary of the study efficacy results is provided in Table 9.
Table 9 - ATAC Efficacy Summary* Intent-To-Treat Population
Hormone Receptor-Positive Subpopulation
Anastrozole
1 mg
(N=3125)
Tamoxifen
20 mg
(N=3116)
Anastrozole
1 mg
(N=2618)
Tamoxifen
20 mg
(N=2598)
Number of Events
Number of Events
- *
- The combination arm was discontinued due to lack of efficacy benefit at 33 months of follow-up.
Disease-free Survival
575
651
424
497
Hazard ratio
0.87
0.78 to 0.97
0.0127
0.83
0.73 to 0.94
0.0049
2-sided 95% CI
p-value
Distant Disease-free Survival
500
530
370
394
Hazard ratio
0.94
0.83 to 1.06
0.93
0.80 to 1.07
2-sided 95% CI
Overall Survival
411
420
296
301
Hazard ratio
0.97
0.85 to 1.12
0.97
0.83 to 1.14
2-sided 95% CI
14.2 First Line Therapy in Postmenopausal Women with Advanced Breast Cancer
Two double-blind, controlled clinical studies of similar design (0030, a North American study and 0027, a predominately European study) were conducted to assess the efficacy of anastrozole compared with tamoxifen as first-line therapy for hormone receptor positive or hormone receptor unknown locally advanced or metastatic breast cancer in postmenopausal women. A total of 1021 patients between the ages of 30 and 92 years old were randomized to receive trial treatment. Patients were randomized to receive 1 mg of anastrozole once daily or 20 mg of tamoxifen once daily. The primary end points for both trials were time to tumor progression, objective tumor response rate, and safety.
Demographics and other baseline characteristics, including patients who had measurable and no measurable disease, patients who were given previous adjuvant therapy, the site of metastatic disease and ethnic origin were similar for the two treatment groups for both trials. The following table summarizes the hormone receptor status at entry for all randomized patients in trials 0030 and 0027.
Table 10 — Demographic and Other Baseline Characteristics Number (%) of subjects
Trial 0030
Trial 0027
Receptor status
anastrozole
1 mg
(n=171)
Tamoxifen
20 mg
(n=182)
anastrozole
1 mg
(n=340)
Tamoxifen
20 mg
(n=328)
151 (88.3)
162 (89.0)
154 (45.3)
144 (43.9)
Unknown
19 (11.1)
20 (11.0)
185 (54.4)
183 (55.8)
For the primary endpoints, trial 0030 showed that anastrozole had a statistically significant advantage over tamoxifen (p=0.006) for time to tumor progression; objective tumor response rates were similar for anastrozole and tamoxifen. Trial 0027 showed anastrozole and tamoxifen had similar objective tumor response rates and time to tumor progression (see Table 11 and Figure 3 and 4)
Table 11 below summarizes the results of trial 0030 and trial 0027 for the primary efficacy endpoints.
Table 11– Efficacy Results of First-line Treatment End point
Trial 0030
Trial 0027
Anastrozole
1 mg
(n=171)
Tamoxifen
20 mg
(n=182)
Anastrozole
1 mg
(n=340)
Tamoxifen
20 mg
(n=328)
Time to progression (TTP)
Median TTP (months)
11.1
5.6
8.2
8.3
Number (%) of subjects
Who progressed
114 (67%)
138 (76%)
249 (73%)
247 (75%)
1.42 (1.15)
1.01 (0.87)
2-sided 95% CI‡
(1.11, 1.82)
(0.85, 1.20)
p-value§
0.006
0.920
Best objective response rate
Number (%) of subjects
36 (21.1%)
31 (17.0%)
112 (32.9%)
107 (32.6%)
1.30 (0.83)
1.01 (0.77)
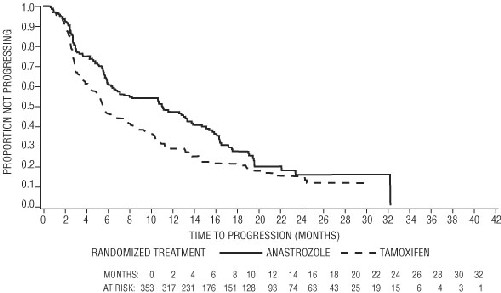
Figure 3 — Kaplan-Meier probability of time to disease progression for all randomized patients (intent to- treat) in Trial 0030
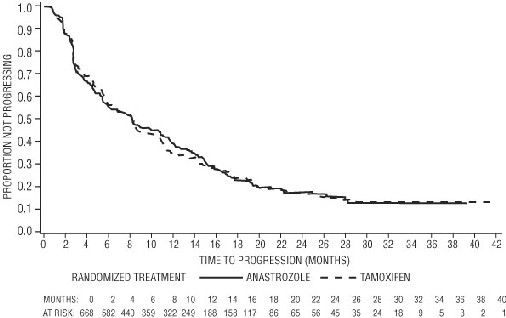
Figure 4 — Kaplan-Meier probability of time to progression for all randomized patients (intent-to-treat) in Trial 0027
Results from the secondary endpoints were supportive of the results of the primary efficacy endpoints. There were too few deaths occurring across treatment groups of both trials to draw conclusions on overall survival differences.
14.3 Second Line Therapy in Postmenopausal Women with Advanced Breast Cancer who had Disease Progression following Tamoxifen Therapy
Anastrozole was studied in two controlled clinical trials (0004, a North American study; 0005, a predominately European study) in postmenopausal women with advanced breast cancer who had disease progression following tamoxifen therapy for either advanced or early breast cancer. Some of the patients had also received previous cytotoxic treatment. Most patients were ER-positive; a smaller fraction were ER-unknown or ER-negative; the ER-negative patients were eligible only if they had had a positive response to tamoxifen. Eligible patients with measurable and non-measurable disease were randomized to receive either a single daily dose of 1 mg or 10 mg of anastrozole. or megestrol acetate 40 mg four times a day. The studies were double-blinded with respect to anastrozole. Time to progression and objective response (only patients with measurable disease could be considered partial responders) rates were the primary efficacy variables. Objective response rates were calculated based on the Union Internationale Contre le Cancer (UICC) criteria. The rate of prolonged (more than 24 weeks) stable disease, the rate of progression, and survival were also calculated.
Both trials included over 375 patients; demographics and other baseline characteristics were similar for the three treatment groups in each trial. Patients in the 0005 trial had responded better to prior tamoxifen treatment. Of the patients entered who had prior tamoxifen therapy for advanced disease (58% in Trial 0004; 57% in Trial 0005), 18% of these patients in Trial 0004 and 42% in Trial 0005 were reported by the primary investigator to have responded. In Trial 0004, 81% of patients were ER-positive, 13% were ER-unknown, and 6% were ER-negative. In Trial 0005, 58% of patients were ER-positive, 37% were ER-unknown, and 5% were ER-negative. In Trial 0004, 62% of patients had measurable disease compared to 79% in Trial 0005. The sites of metastatic disease were similar among treatment groups for each trial. On average, 40% of the patients had soft tissue metastases; 60% had bone metastases; and 40% had visceral (15% liver) metastases.
Efficacy results from the two studies were similar as presented in Table 12. In both studies there were no significant differences between treatment arms with respect to any of the efficacy parameters listed in the table below.
Table 12 – Efficacy Results of Second-line Treatment Anastrozole
1 mg
Anastrozole
10 mg
Megestrol
Acetate
160 mg
- *
- Surviving Patients
Trial 0004
(N. America)
(N=128)
(N=130)
(N=128)
Median Follow-up (months)*
31.3
30.9
32.9
Median Time to Death (months)
29.6
25.7
26.7
2 Year Survival Probability (%)
62.0
58.0
53.1
Median Time to Progression (months)
5.7
5.3
5.1
Objective Response
(all patients ) (%)
12.5
10.0
10.2
Stable Disease for >24 weeks (%)
35.2
29.2
32.8
Progression (%)
86.7
85.4
90.6
Trial 0005
(Europe, Australia, S. Africa)
(N=135)
(N=118)
(N=125)
Median Follow-up (months)*
31.0
30.9
31.5
Median Time to Death (months)
24.3
24.8
19.8
2 Year Survival Probability (%)
50.5
50.9
39.1
Median Time to Progression (months)
4.4
5.3
3.9
Objective Response
(all patients) (%)
12.6
15.3
14.4
Stable Disease for >24 weeks (%)
24.4
25.4
23.2
Progression (%)
91.9
89.8
92.0
When data from the two controlled trials are pooled, the objective response rates and median times to progression and death were similar for patients randomized to anastrozole 1 mg and megestrol acetate. There is, in this data, no indication that anastrozole 10 mg is superior to anastrozole 1 mg.
Table 13– Pooled Efficacy Results of Second-line Treatment Trials 0004 & 0005
(Pooled Data)
Anastrozole
1 mg
N=263
Anastrozole
10 mg
N=248
Megestrol Acetate
160 mg
N=253
Median Time to
Death (months)
26.7
25.5
22.5
2 Year Survival
Probability (%)
56.1
54.6
46.3
Median Time to
Progression
4.8
5.3
4.6
Objective Response
(all patients) (%)
12.5
12.5
12.3
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
17.1 Pregnancy
Patients should be advised that anastrozole may cause fetal harm. They should also be advised that anastrozole tablet is not for use in premenopausal women; therefore, if they become pregnant they should stop taking anastrozole tablets and immediately contact their doctor.
17.2 Allergic (Hypersensitivity) Reactions
Patients should be informed of the possibility of serious allergic reactions with swelling of the face, lips, tongue and/or throat (angioedema) which may cause difficulty in swallowing and/or breathing and to immediately report this to their doctor.
17.3 Ischemic Cardiovascular Events
Patients with pre-existing ischemic heart disease should be informed that an increased incidence of cardiovascular events has been observed with anastrozole use compared to tamoxifen use.
17.4 Bone Effects
Patients should be informed that anastrozole lowers the level of estrogen. This may lead to a loss of the mineral content of bones, which might decrease bone strength. A possible consequence of decreased mineral content of bones is an increase in the risk of fractures.
-
17.7 FDA-Approved Patient Labeling
Manufactured For:
Accord Healthcare, Inc.,
1009, Slater Road,
Suite 210-B,
Durham, NC 27703,
USA.Manufactured By:
Intas Pharmaceuticals Limited,
Plot No.: 457, 458,
Village – Matoda,
Bavla Road, Ta. - Sanand,
Dist.- Ahmedabad – 382 210.
India.Repackaged By:
Rebel Distributors Corp
Thousand Oaks, CA 91320
PATIENT INFORMATION
Anastrozole Tablets
(an as' troe zole)Read the information that comes with anastrozole tablets before you start taking it and each time you get a refill. The information may have changed. This leaflet does not take the place of talking with your doctor about your medical condition or treatment. Talk with your doctor about anastrozole tablets when you start taking it and at regular checkups.
What is an anastrozole tablet?
Anastrozole tablet is a prescription medicine used in women who have finished menopause (“the change of life”) for:
- treatment of early breast cancer
- after surgery, with or without radiation
- in women whose breast cancer is hormone receptor-positive
- first treatment of locally advanced or metastatic breast cancer, in women whose breast cancer is hormone receptor-positive or the hormone receptors are not known.
- treatment of advanced breast cancer, if the cancer has grown, or the disease has spread after tamoxifen therapy.
Anastrozole tablet does not work in women with breast cancer who have not finished menopause (premenopausal women).
Who should not take anastrozole tablets?
Do not take anastrozole tablet if you:
- are pregnant, think you may be pregnant, or plan to get pregnant. Anastrozole tablets may harm your unborn child. If you become pregnant while taking anastrozole tablets, tell your doctor right away.
- have not finished menopause (are premenopausal)
- are allergic to any of the ingredients in anastrozole tablets. See the end of this leaflet for a list of the ingredients in anastrozole tablets.
- are a man or child
What is the most important information I should know about anastrozole tablets? Anastrozole tablets may cause serious side effects including:
- Heart disease. Women with early breast cancer, who have a history of blockages in heart arteries (ischemic heart disease) and who take anastrozole tablets may have a slight increase in this type of heart disease compared to similar patients who take tamoxifen.
- Stop taking anastrozole tablets and call your doctor right away if you have chest pain or shortness of breath. These can be symptoms of heart disease.
- Osteoporosis (bone softening and weakening). Anastrozole tablets lowers estrogen in your body, which may cause your bones to become softer and weaker. This can increase your chance of fractures, specifically of the spine, hip and wrist. Your doctor may order a test for you called a bone mineral density study before you start taking anastrozole tablets and during treatment with anastrozole tablets as needed.
What should I tell my doctor before taking anastrozole tablets?Anastrozole tablets may not be right for you. Before taking anastrozole tablets, tell your doctor about all your medical conditions, including if you:
- have not finished menopause. Talk to your doctor if you are not sure. See “Who should not take anastrozole tablets?”
- have had a previous heart problem
- have a condition called osteoporosis
- have high cholesterol
- are pregnant, planning to become pregnant, or breast feeding. See “Who should not take anastrozole tablets?”
- are nursing a baby. It is not known if anastrozole tablets passes into breast milk. You and your doctor should decide if you will take anastrozole tablets or breast feed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Especially tell your doctor if you take:
- Tamoxifen. You should not take anastrozole tablets with tamoxifen. Taking tamoxifen with anastrozole tablets may lower the amount of anastrozole tablets in your blood and may cause anastrozole tablets not to work as well.
- Medicines containing estrogen. Anastrozole tablets may not work if taken with one of these medicines:
- hormone replacement therapy
- birth control pills
- estrogen creams
- vaginal rings
- vaginal suppositories
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist each time you get a new medicine.
How should I take anastrozole tablets?
- Take anastrozole tablets exactly as prescribed by your doctor. Keep taking anastrozole tablets for as along as your doctor prescribes it for you.
- Take one anastrozole tablet each day
- Anastrozole tablets can be taken with or without food.
- If you miss a dose, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Take your next regularly scheduled dose. Do not take two doses at the same time.
- If you have taken more anastrozole tablets than your doctor has prescribed, contact your doctor right away. Do not take any additional anastrozole tablets until instructed to do so by your doctor.
Talk with your doctor about any health changes you have while taking anastrozole tablets.
What are possible side effects of anastrozole tablets?
Anastrozole tablets can cause serious side effects including:
- See “What is the most important information I should know about anastrozole tablets?”
- increased blood cholesterol (fat in the blood). Your doctor may check your cholesterol while you take anastrozole tablets therapy.
- skin reactions. Stop taking anastrozole tablets and call your doctor right away if you get any skin lesions, ulcers, or blisters.
- severe allergic reactions. Get medical help right away if you have:
- swelling of the face, lips, tongue, or throat.
- trouble swallowing
- trouble breathing
- liver problems. Anastrozole tablets can cause inflammation of the liver and changes in blood tests of the liver function. Your doctor may monitor you for this. Stop taking anastrozole tablets and call your doctor right away if you have any of these signs or symptoms of a liver problem:
- a general feeling of not being well
- yellowing of the skin or whites of the eyes
- pain on the right side of your abdomen
Common side effects in women taking Anastrozole tablet include:
- hot flashes
- weakness
- joint pain
- carpal tunnel syndrome (tingling, pain, coldness, weakness in parts of the hand)
- pain
- sore throat
- mood changes
- high blood pressure
- depression
- nausea and vomiting
- thinning of the hair (hair loss)
- rash
- back pain
- sleep problems
- bone pain
- headache
- swelling
- increased cough
- shortness of breath
- lymphedema (build up of lymph fluid in the tissues of your affected arm)
- trigger finger (a condition in which one of your fingers or your thumb catches in a bent position)
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
HOW SHOULD I STORE ANASTROZOLE TABLETS?
- Store anastrozole tablets at 68°F to 77°F (20°C to 25°C).
- Keep anastrozole tablets and all medicines out of the reach of children.
General information about anastrozole tablets.
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not take anastrozole tablets for a condition for which it was not prescribed. Do not give anastrozole tablets to other people, even if they have the same symptoms you have. It may harm them.
This patient information leaflet summarizes the most important information about anastrozole tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about anastrozole tablets that is written for health professionals.
What are the ingredients in anastrozole tablets?
Active ingredient: anastrozole
Inactive ingredients: lactose monohydrate, magnesium stearate, hypromellose, macrogol, povidone, sodium starch glycolate, and titanium dioxide.
Manufactured For:
Accord Healthcare, Inc.,
1009, Slater Road,
Suite 210-B,
Durham, NC 27703,
USA.Manufactured By:
Intas Pharmaceuticals Limited,
Plot No.: 457, 458,
Village – Matoda,
Bavla Road, Ta. - Sanand,
Dist.- Ahmedabad – 382 210.
India.Repackaged By:
Rebel Distributors Corp
Thousand Oaks, CA 91320
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANASTROZOLE
anastrozole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42254-161(NDC:16729-035) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANASTROZOLE (UNII: 2Z07MYW1AZ) (ANASTROZOLE - UNII:2Z07MYW1AZ) ANASTROZOLE 1 mg Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape ROUND (round biconvex) Size 6mm Flavor Imprint Code AHI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42254-161-30 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090568 06/28/2010 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK


