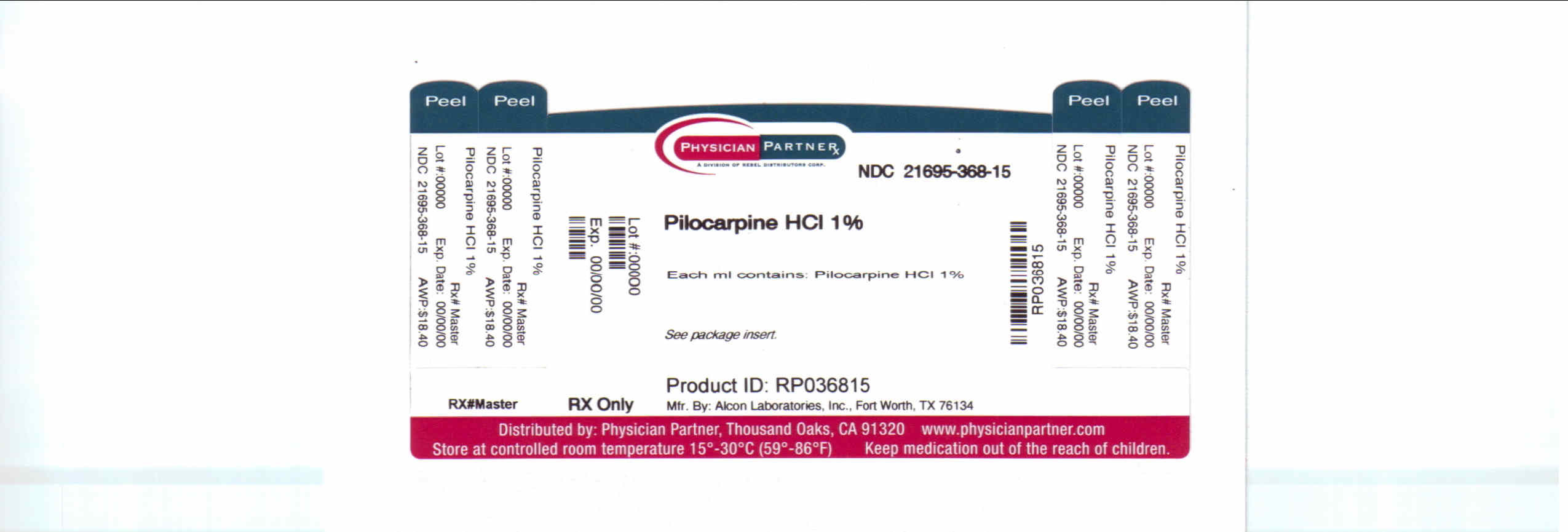
Label: PILOCARPINE HYDROCHLORIDE solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-368-01 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 61314-203
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 15, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
Pilocarpine Hydrochloride is a cholinergic prepared as a sterile topical ophthalmic solution. The active ingredient is represented by the chemical structural formula:
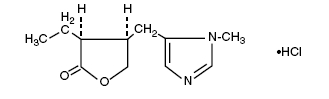
C11H16N2O2•HCl
Mol. Wt. 244.72
2(3H)-Furanone, 3-ethyldihydro-4-[(1-methyl-1H-imidazol-5-yl)methyl] - monohydrochloride, [3S-cis] -
Pilocarpine HCI 0.5%
EACH mL CONTAINS: ACTIVE: Pilocarpine Hydrochloride 10 mg (1%); INACTIVES: Hypromellose, boric acid, sodium citrate, sodium chloride, Purified Water. Sodium Hydroxide (added to adjust pH).
PRESERVATIVE ADDED: Benzalkonium Chloride 0.01%.Pilocarpine HCI 4%
EACH mL CONTAINS: ACTIVE: Pilocarpine Hydrochloride 40 mg (4%); INACTIVES: Hypromellose, Monobasic Sodium Phosphate, Edetate Disodium, Dibasic Sodium Phosphate, Purified Water. Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH (3.5 - 5.5). PRESERVATIVE ADDED: Benzalkonium Chloride 0.01%.
- CLINICAL PHARMACOLOGY:
-
INDICATIONS AND USAGE:
Pilocarpine hydrochloride can be used in the medical management of glaucoma, especially open-angle glaucoma, in those cases in which the intraocular pressure can be controlled adequately by the topical administration of pilocarpine. In acute (closed-angle) glaucoma, pilocarpine hydrochloride may be used alone, or in combination with other cholinergic agents or carbonic anhydrase inhibitors, to relieve tension prior to emergency surgery.
Patients may be maintained on pilocarpine hydrochloride as long as intraocular pressure is controlled and there is no deterioration in the visual fields. The choice of concentration should be determined by the severity of the condition and the response of the patient. Pilocarpine hydrochloride is also indicated to counter the effects of cycloplegics and mydriatics following surgery or ophthalmoscopic examination.
- CONTRAINDICATIONS:
- WARNINGS:
-
PRECAUTIONS:
This pilocarpine-induced miosis may cause difficulty in dark adaptation. The patient should exercise caution when involved in night driving or other hazardous activities in poor light.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
There have been no long-term studies done using pilocarpine in animals to evaluate carcinogenic potential.
-
ADVERSE REACTIONS:
OCULAR: Transient symptoms of stinging and burning may occur. Ciliary spasm, conjunctival vascular congestion, temporal or supraorbital headache, lacrimation and induced myopia may occur. This is especially true in younger individuals who have recently started administration. Reduced visual acuity in poor illumination is frequently experienced by older individuals and individuals with lens opacity. Miotic agents may also cause retinal detachment; thus, care should be exercised with all miotic therapy especially in young myopic patients. Lens opacity may occur with prolonged use of pilocarpine.
SYSTEMIC: Systemic reactions following topical administration, although extremely rare, have included hypertension, tachycardia, bronchiolar spasm, pulmonary edema, salivation, sweating, nausea, vomiting, and diarrhea.
-
DOSAGE AND ADMINISTRATION:
The initial dose is one or two drops in the affected eye(s). This may be repeated up to three or four times daily or as directed by a physician. The frequency of instillation and concentration of pilocarpine hydrochloride ophthalmic solution are determined by the severity of the glaucoma and miotic response of the patient. Individuals with heavily pigmented irides may require higher strengths.
During acute phases, the miotic must be instilled into the unaffected eye to prevent an attack of angle-closure glaucoma.
DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT.
-
HOW SUPPLIED
Pilocarpine Hydrochloride Ophthalmic Solution USP is supplied in 15 ml plastic DROP-TAINER* dispensers.
*DROP-TAINER is a registered trademark of Alcon Manufacturing Ltd.
NOT FOR INJECTION.
FOR OPHTHALMIC USE ONLY.
STORAGE: Store between 8°- 27°C (46°- 80°F).
KEEP OUT OF REACH OF CHILDREN.
Manufactured by: ALCON LABORATORIES, INC, Fort Worth, TX 76134
Distributed by: FALCON PHARMACEUTICALS LTD., Fort Worth, TX 76134
Repackaged by: REBEL DISTRIBUTORS CORP, Thousand Oaks, CA 91320
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PILOCARPINE HYDROCHLORIDE
pilocarpine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-368(NDC:61314-203) Route of Administration CONJUNCTIVAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PILOCARPINE HYDROCHLORIDE (UNII: 0WW6D218XJ) (PILOCARPINE - UNII:01MI4Q9DI3) PILOCARPINE HYDROCHLORIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BORIC ACID (UNII: R57ZHV85D4) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-368-01 1 in 1 CARTON 1 15 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA200890 06/22/2010 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK