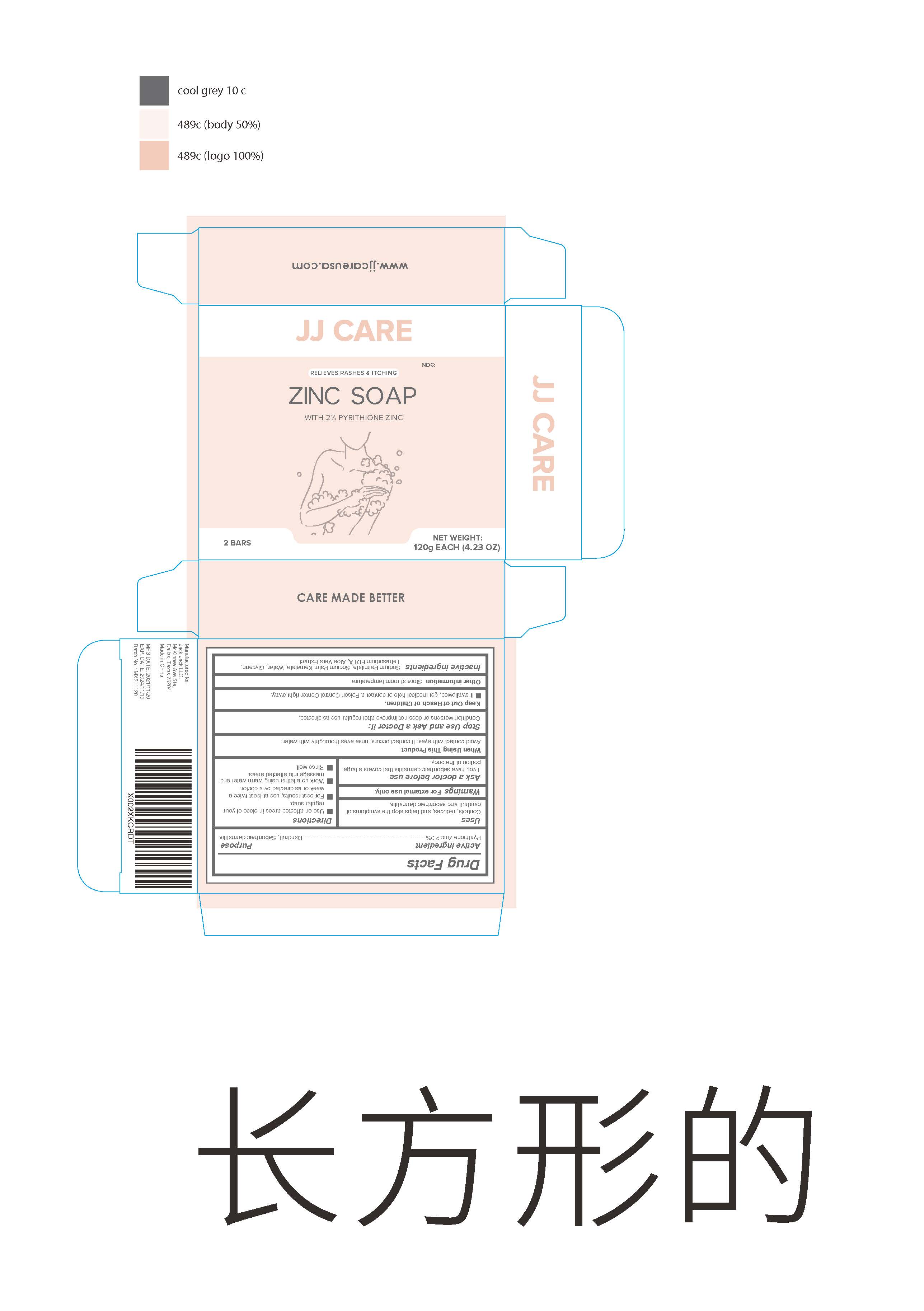
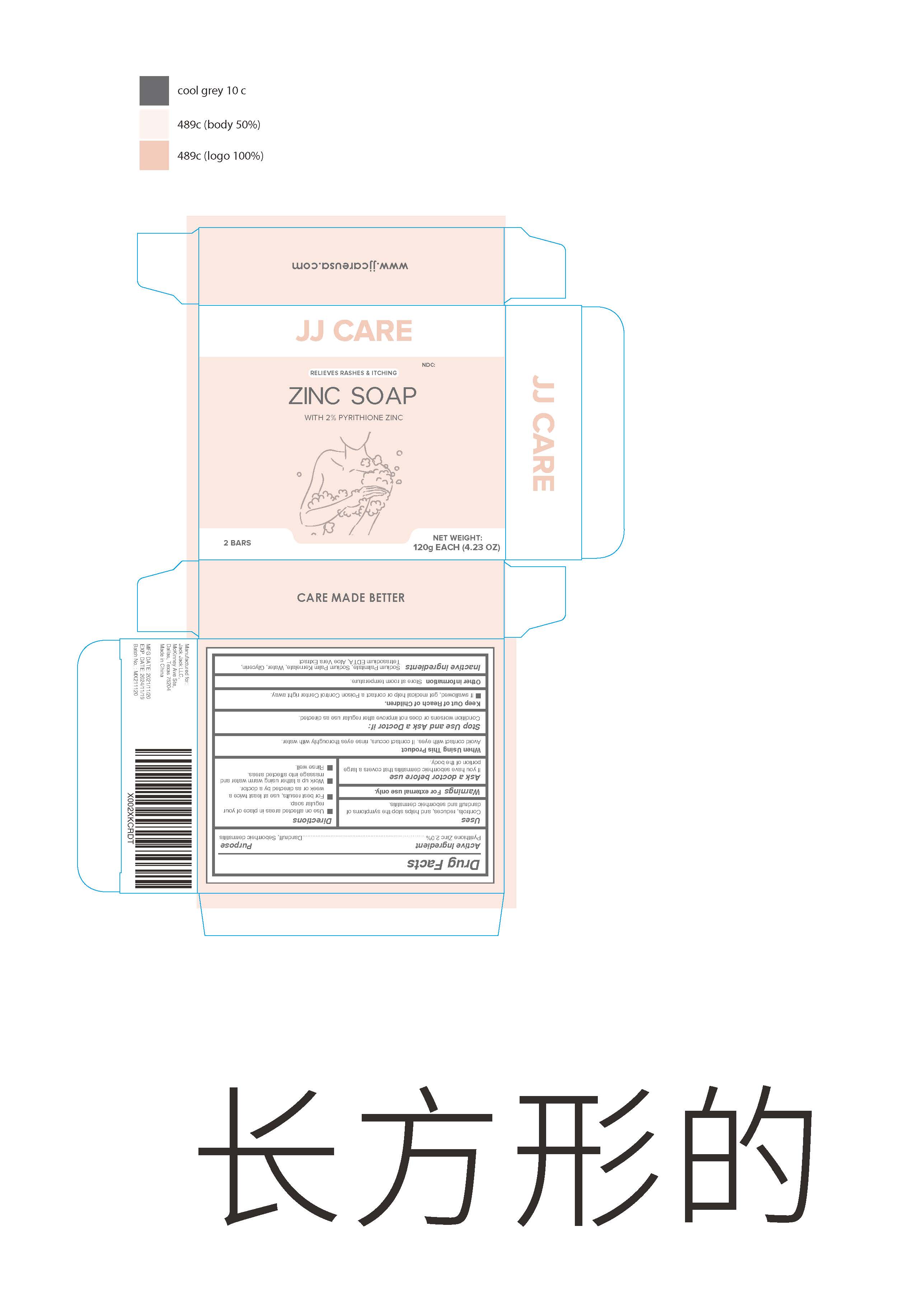
ZINC- zinc soap soap
Meixin Beauty & Health Care Products Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
78565-007
Zinc soap
When using this product Avoid contact with eyes. If contact occurs , rinse eyes thoroughly with water
Keep Out of Reach of Children
If swallowed , get medical help or contact a Poison Control Center right away
Directions
Use on affected areas in place of your regular soap.
For best results use at least twice a week or as directed by a doctor.
Work up a lather using warm water and massage into affected areas.
Rinse well.
| ZINC
zinc soap soap |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
 |
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Meixin Beauty & Health Care Products Co., Ltd. (421458605) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Meixin Beauty & Health Care Products Co., Ltd. | 421458605 | manufacture(78565-007) | |
Revised: 12/2021
Document Id: d3f42cbc-0acd-0968-e053-2a95a90ad5bd
Set id: d1e9e9d3-99ae-86b1-e053-2995a90a136a
Version: 4
Effective Time: 20211225
Meixin Beauty & Health Care Products Co., Ltd.