SULFUR- sulfur soap soap
Meixin Beauty & Health Care Products Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
78565-106
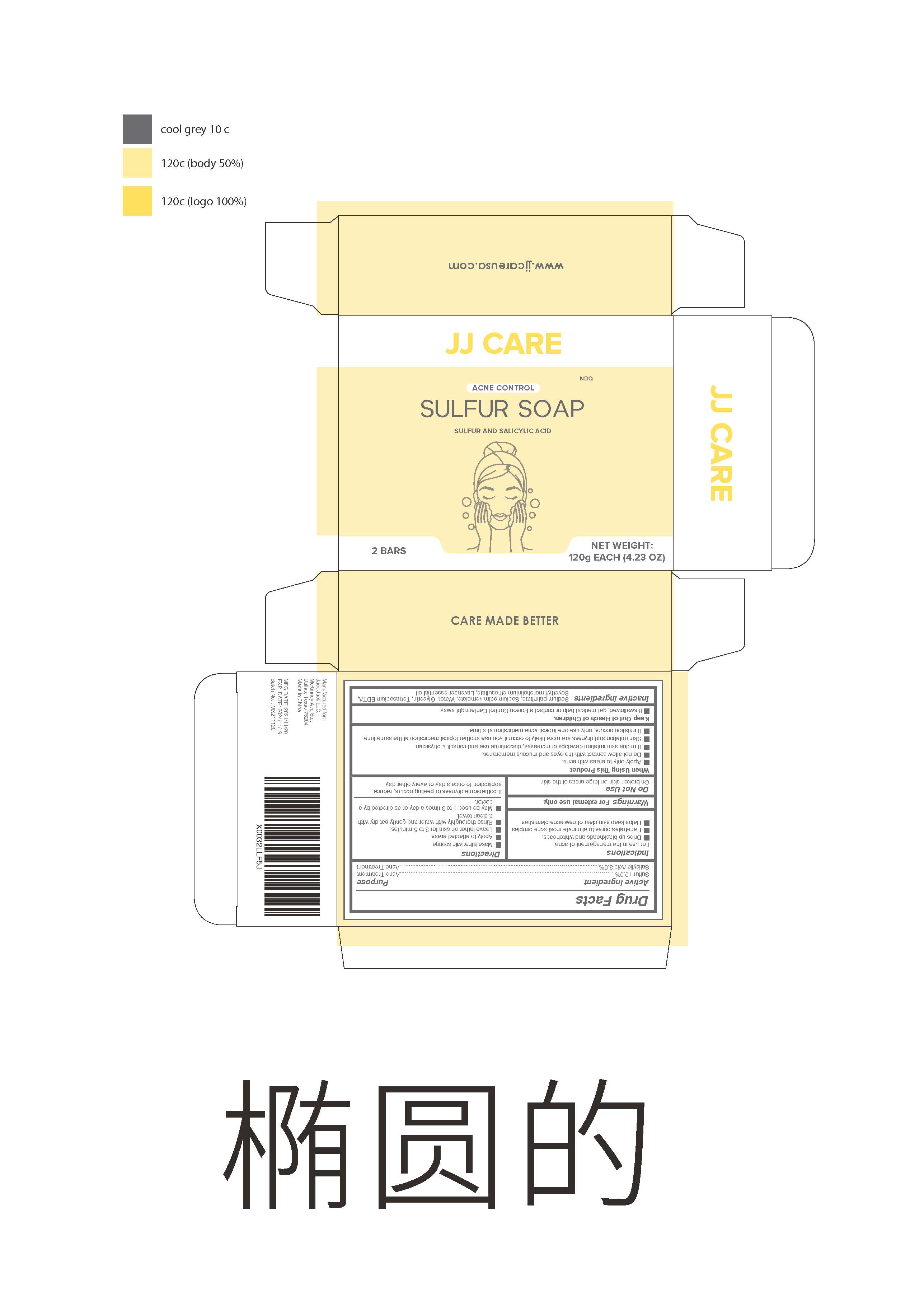
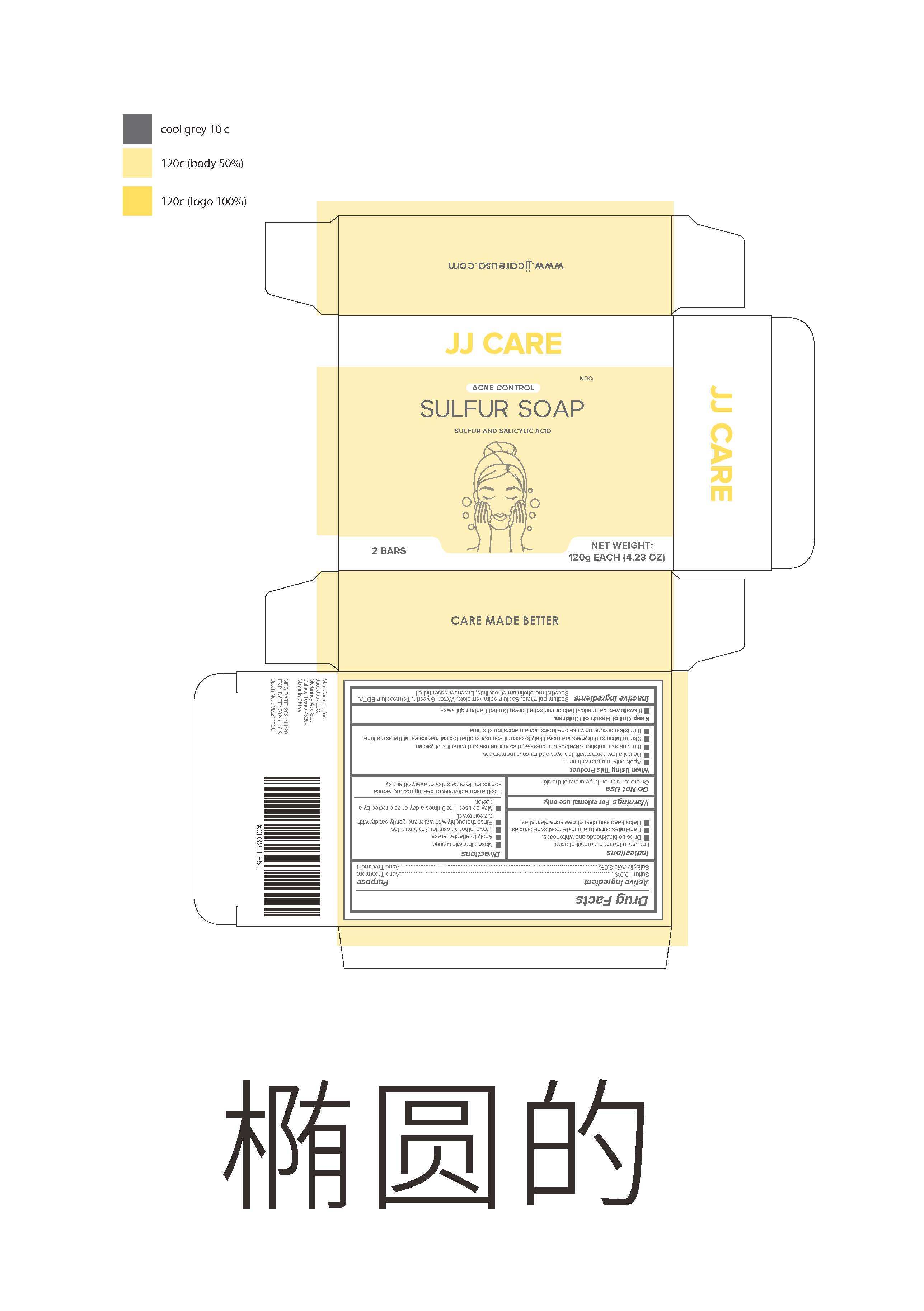
Sulfur soap
When Using This Product
Apply only to areas with acne.
Do not allow contact with the eyes and mucous membranes.
If undue skin irritation develops or increases , discontinue use and consult a physician.
Skin irritation and dryness are more likely to occur if you use another topical medication at the same time.
If irritation occurs , only use one topical acne medication at a time.
Keep Out of Reach of Children.
If swallowed , get medical help or contact a Poison Control Center right away.
Directions
Make lather with sponge.
Apply to affected areas.
Leave lather on skin for 3 to 5 minutes.
Rinse thoroughly with water and gently pat dry with a clean toweI.
May be used 1 to 3 times a day or as directed by a doctor
Inactive ingredients
Sodium palmitate, Sodium palm kernelate, Water , Glycerin , Tetrasodium EDTA ,Soyethyl morpholinium ethosulfate , Lavender essential oil
| SULFUR
sulfur soap soap |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
 |
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Meixin Beauty & Health Care Products Co., Ltd. (421458605) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Meixin Beauty & Health Care Products Co., Ltd. | 421458605 | manufacture(78565-106) | |