PANCREAPOWDER PLUS- pancreatic enzyme concentrate plus fat soluble vitamins powder
Henry Schein Animal Health
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
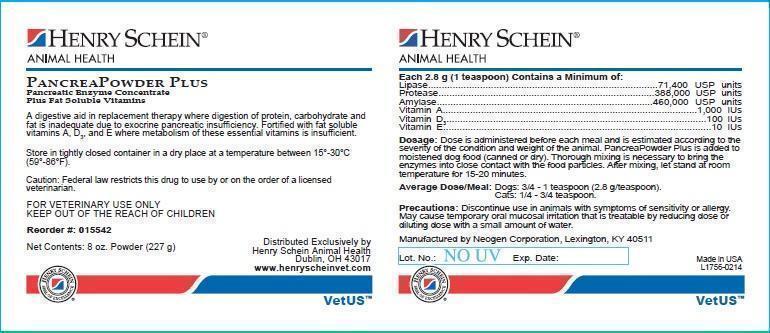
PancreaPowder Plus
Pancreatic Enzyme Concentrate Plus Fat Soluble Vitamins
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Each 2.8 grams (1 teaspoon) contains a minimum of:
Lipase..............................71,400 USP units
Protease.........................388,000 USP units
Amylase..........................460,000 USP units
Vitamin A...........................1,000 IU
Vitamin D3.........................100 IU
Vitamin E...........................10 IU
Precautions:
Discontinue use in animals with symptoms of sensitivity or allergy. May cause temporary oral mucosal irritation that is treatable by reducing dose or diluting dose with a small amount of water.
Indications:
A digestive aid in replacement therapy where digestion of protein, carbohydrate and fat is inadequate due to exocrine pancreatic insufficiency. Fortified with fat soluble vitamins A, D3 and E where metabolism of these essential vitamins is insufficient.
Dosage:
Dose is administered before each meal and is estimated according to the severity of the condition and weight of the animal. PancreaPowder Plus is added to moistened food (canned or dry). Thorough mixing is necessary to bring the enzymes into close contact with the food particles. After mixing, let stand at room temperature for 15-20 minutes.
Average dose/meal:
Dogs: 3/4-1 teaspoon (2.8g/teaspoon)
Cats: 1/4-3/4 teaspoon
Distributed Exclusively by:
Henry Schein Animal Health
Dublin, OH 43017
www.henryscheinvet.com
Manufactured by: Neogen Corporation,
Lexington, KY 40511
PRINCIPAL DISPLAY PANEL - 4 oz Bottle
NDC: 11695-1237-4
PancreaPowder Plus
Pancreatic Enzyme Concentrate Plus Fat Soluble Vitmains
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Henry Schein® Animal Health
Reorder #: 015541
Net Contents: 4 oz Powder (113 g)

PRINCIPAL DISPLAY PANEL - 8 oz Bottle
NDC: 11695-1237-5
PancreaPowder Plus
Pancreatic Enzyme Concentrate Plus Fat Soluble Vitamins
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Reorder #: 015542 Henry Schein® Animal Health
Net Contents: 8 oz (227 g)
PRINCIPAL DISPLAY PANEL - 12 oz Bottle
NDC: 11695-1237-6
PancreaPowder Plus
Pancreatic Enzyme Concentrate Plus Fat Soluble Vitamins
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Reorder #: 015543
Henry Schein® Animal Health
Net Contents: 12 oz (340 g)

| PANCREAPOWDER PLUS
pancreatic enzyme concentrate plus fat soluble vitamins powder |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Henry Schein Animal Health (603750329) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Neogen Corporation-Mercer | 042125879 | analysis, manufacture, label | |