Label: GENTAMICIN SULFATE ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-1040-1 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0168-0044
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 7, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Gentamicin sulfate is a water soluble antibiotic of the aminoglycoside group.
Gentamicin Sulfate Ophthalmic Ointment USP is a sterile ointment for topical ophthalmic use. Each gram contains gentamicin sulfate (equivalent to 3 mg gentamicin) in a base of white petrolatum and mineral oil.
Gentamicin is obtained from cultures of Micromonospora purpurea. Gentamicin sulfate is a mixture of the sulfate salts of gentamicin C1, C2 and C1A. All three components appear to have similar antimicrobial activities. Gentamicin sulfate occurs as a white powder and is soluble in water and insoluble in alcohol. The structural formula is as follows:
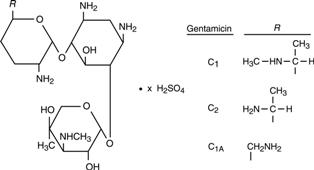
-
CLINICAL PHARMACOLOGY
Microbiology: Gentamicin sulfate is active in vitro against many strains of the following microorganisms:
Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Streptococcus pneumoniae, Enterobacter aerogenes, Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Neisseria gonorrhoeae, Pseudomonas aeruginosa, and Serratia marcescens. -
INDICATIONS AND USAGE
Gentamicin Sulfate Ophthalmic Ointment is indicated in the topical treatment of ocular bacterial infections, including conjunctivitis, keratitis, keratoconjunctivitis, corneal ulcers, blepharitis, blepharoconjunctivitis, acute meibomianitis, and dacryocystitis caused by susceptible strains of the following microorganisms:
Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Streptococcus pneumoniae, Enterobacter aerogenes, Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Neisseria gonorrhoeae, Pseudomonas aeruginosa, and Serratia marcescens. - CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General: Prolonged use of topical antibiotics may give rise to overgrowth of non-susceptible organisms including fungi. Bacterial resistance to gentamicin may also develop. If purulent discharge, inflammation or pain becomes aggravated, the patient should discontinue use of the medication and consult a physician.
If irritation or hypersensitivity to any component of the drug develops, the patient should discontinue use of this preparation, and appropriate therapy should be instituted.
Ophthalmic ointments may retard corneal healing.
Information For Patients: To avoid contamination, do not touch tip of container to the eye, eyelid, or any surface.
Carcinogenesis, Mutagenesis and Impairment of Fertility: There are no published carcinogenicity or impairment of fertility studies on gentamicin. Aminoglycoside antibiotics have been found to be non-mutagenic.
Pregnancy:Teratogenic effects - Pregnancy Category C. Gentamicin has been shown to depress body weights, kidney weights, and median glomerular counts in newborn rats when administered systemically to pregnant rats in daily doses approximately 500 times the maximum recommended ophthalmic human dose. There are no adequate and well-controlled studies in pregnant women. Gentamicin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pediatric Use: Safety and effectiveness in neonates have not been established.
-
ADVERSE REACTIONS
Bacterial and fungal corneal ulcers have developed during treatment with gentamicin ophthalmic preparations.
The most frequently reported adverse reactions are ocular burning and irritation upon drug instillation, non-specific conjunctivitis, conjunctival epithelial defects, and conjunctival hyperemia.
Other adverse reactions which have occurred rarely are allergic reactions, thrombocytopenic purpura, and hallucinations.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Gentamicin Sulfate Ophthalmic Ointment USP (equivalent to 3 mg gentamicin) is supplied as follows:
NDC 54868-1040-1 3.5 gram tubes
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F)
[see USP Controlled Room Temperature].E. FOUGERA & CO.
a division of Altana Inc.
MELVILLE, NEW YORK 11747I24438A
R4/03
#82
Relabeling of "Additional Barcode" by:
Physicians Total Care, Inc.
Tulsa, OK 74146
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GENTAMICIN SULFATE
gentamicin sulfate ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-1040(NDC:0168-0044) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GENTAMICIN SULFATE (UNII: 8X7386QRLV) (GENTAMICIN - UNII:T6Z9V48IKG) GENTAMICIN 3 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-1040-1 3.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065024 09/21/1994 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel


